Photoelectrochemical determination of intrinsic kinetics of photoelectrocatalysis processes at {001} faceted anatase TiO2 photoanodes†
Tao Suna,
Yun Wang*a,
Mohammad Al-Mamuna,
Haimin Zhanga,
Porun Liua and
Huijun Zhao*ab
aCentre for Clean Environment and Energy, Griffith School of Environment, Griffith university, Gold Coast Campus, QLD 4222, Australia. E-mail: yun.wang@griffith.edu.au; h.zhao@griffith.edu.au; Fax: +61 1 55528067; Tel: +61 1 55528261
bCentre for Environmental and Energy Nanomaterials, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei 230031, China
First published on 16th January 2015
Abstract
The understanding of the intrinsic degradation kinetics of organic species at the reactive anatase TiO2 (001) surfaces under operational conditions is essential for the development of photoelectrocatalytic treatment of wastewater or polluted air. In this study, the intrinsic degradation kinetics of oxalic acid on the anatase (001) surface is successfully investigated by using a facile photoelectrochemical (PEC) method. A double-layered TiO2 photoanode with mainly anatase {001} facets exposed is purposely designed for the PEC measurements. The results reveal that the adsorption of oxalic acid follows the Langmuir adsorption model within the investigated concentration range. The PEC degradation profile can be fitted by two different first-order kinetic processes. The measured rate constant for the fast degradation processes is five times higher than that of the slow processes. The results confirm that the anatase TiO2 with exposed {001} facets possesses a higher reactivity than that of {101} faceted anatase TiO2.
Introduction
Photo-degradation of organic species by semiconductors, such as titanium dioxides (TiO2), is one of the attractive approaches for the treatment of wastewater and polluted air.1,2 A meaningful measurement of intrinsic kinetics of photoelectrocatalytic reactions can provide insightful information on the photocatalytic degradation process, critically important for photocatalysis applications.3–6 However, most of the reported photoelectrocatalytic kinetics studies are focused on the apparent reaction kinetics, which are a collective result of many factors. In addition, the apparent kinetics measurement methods are inadequate for directly investigating the overall kinetics of photocatalysis because their inability to determine the instantaneous reaction rate constant. Moreover, the degradation kinetics of strong adsorbates on a highly photoactive surface is difficult because the initial rate cannot be precisely defined due to the adsorption of analytes on the electrode surface. Thus, it is difficult to establish a precise relationship between the adsorption strength of organic species and the apparent reaction rates. To this end, it becomes rather challenging to experimentally measure the adsorption properties under operational conditions. One breakthrough is the introduction of a simple, rapid and accurate photoelectrochemical (PEC) method to directly and spontaneously characterize thermodynamic and instant kinetic properties for adsorption and degradation of organic species at the surface of photocatalysts.7–12 Another attraction of PEC measurement is that it allows the investigation of intrinsic degradation kinetics without the influence of other factors such as mass transfer and the reduction processes.9,13,14 Therefore, the intrinsic reaction rates obtained from PEC measurements can be strongly correlated to the adsorption strength of adsorbates under operational conditions.It is well known that the photoelectrocatalytic reactivity of inorganic crystals is also governed by the surface geometric structures.15–20 Among all TiO2 surfaces, the anatase (001) surface has been theoretically predicated to possess high reactivity.9,12,21–23 However, the majority of the reported studies on photocatalytic degradation kinetics of organic species at the anatase (001) surface have been performed by the measurement of the rate of the reactant disappearance to determine the apparent rate of reaction.20,24–26 Although there have been some PEC studies of the oxidative degradation of organic species at different TiO2 surfaces,9,11,12,23,27 to our knowledge, there is no report on determination of the intrinsic degradation kinetics of organic species at the reactive anatase (001) surface.
The photocatalytic degradation of organics is essentially an interfacial electron transfer process for which the reactant adsorption at the catalyst surface plays a critical role to affect the kinetic behaviour of the reaction system.10 Nevertheless, the precise determination of adsorption at a catalysts surface by traditional methods is tedious, time consuming and inaccurate.9,27 Additionally, the adsorption of organics at the {001} faceted anatase TiO2 surface has not yet been reported. Therefore, it would be attractive to determine the adsorption properties of {001} faceted anatase TiO2 in a simple, rapid and effective manner.
In this study, a PEC method8–10,12–14,28,29 is employed to quantitatively investigate the surface adsorption properties and the kinetic processes of photocatalytic degradation of oxalic acid at the {001} facet dominated anatase TiO2 photoanode by determining the adsorption constant and the instantaneous inherent rate constant.
Experimental section
Preparation of TiO2 photoanodes
In this study, three types of photoanodes were fabricated by immobilising TiO2 onto a FTO conductive substrate (45 mm × 15 mm × 2 mm).The dense film photoanode (denoted as DF photoanode) was fabricated using a dip-coating method. An aqueous colloidal TiO2 was firstly prepared by hydrolysing titanium butoxide.30 The resultant colloidal solution contains ca. 60 g dm−3 of TiO2 solid with particle sizes ranging from 8 to 10 nm. The carbowax (30% w/w based on the solid weight of the TiO2 colloids) was then added to form the coating solution. The FTO glass slides were cleaned by deionized water, 2-propanol and acetone mixed solution before using as the substrate. The cleaned slides were immersed in coating solution and then withdrawn at a speed of 2 mm s−1 to control the TiO2 film thickness. The coated substrates were dried in air and then calcinated at 450 °C for 2 hours in a muffle furnace (Fig. S1, ESI†). The thickness of the DF photoanode was measured to be 600 nm by the profilometer.
The {001} faceted single-layer anatase TiO2 film photoanode (denoted as A001 photoanode) was fabricated using a reported method.24 Briefly, 2.808 g NaCl was mixed with 60 ml 0.04 M TiF4 solution, which was prepared by adding 0.297 g of TiF4 into 60 ml of 0.01 M HCl, in a Teflon-lined stainless steel autoclave (100 ml volume). The solution was stirred at ambient conditions for 10 min to ensure a complete dissolution. FTO glasses were cleaned by washing with deionized water, 2-propanol and acetone, respectively before being immersed into the solution and placed against the Teflon-liner wall at an angle with the conducting side facing down. The autoclave was heated to 200 °C in an oven. The hydrothermal reaction duration has been demonstrated to greatly affect the morphology of TiO2 crystals.31 Four-hour hydrothermal duration was set in this study because at which the products possess the highest percentage of exposed {001} facets (Fig. S2, ESI†). The resultant coatings were then removed from the autoclave and rinsed with deionized water and dried in air at 50 °C for 30 minutes. The A001 photoanode was obtained by calcinating the resultant coatings in a tube furnace at 450 °C for 2 hours in air with a heating rate of 5 °C min−1.
A two-step procedure was used to fabricate the double-layered photoanode with a dense bottom layer and {001} faceted anatase TiO2 top layer (denoted as DL photoanode). The first step was to coat a dense film onto the FTO substrate with the same procedure used for fabrication of DF photoanode. The DL photoanode was fabricated using the resultant dense film coated substrate as the ‘substrate’ to further grow the top A001 layer with the same hydrothermal method used for fabrication of A001 photoanode.
Characterization
The obtained products were comprehensively characterized by Scanning electron microscopy (SEM, JSM-6300F), transmission electron microscopy (TEM, Philips F20), and X-ray diffraction (XRD, Shimadzu XRD-6000 diffractometer).PEC measurements
All PEC measurements were conducted in a three-electrode PEC cell with a quartz window (0.785 cm2) for ultraviolet (UV) illumination.1,14,27,32,33 The TiO2 photoanodes were used as the working electrode, and a platinum mesh and a saturated Ag/AgCl were used as the counter and reference electrodes, respectively. A 0.1 M NaNO3 solution was used as the electrolyte. A voltammograph (CV-27, BAS) was employed for the application of potential bias. The photocurrent signals and potential were recorded using a Maclab 400 interface (AD Instruments). UV illumination was carried out by a 150 W Xenon arc lamp light with focusing lenses (HF-200W-95, Beijing Optical Instruments). To avoid the electrolyte being heated-up by the infrared light, a UV-band pass filter (UG 5, Avotronics Pty. Ltd.) was utilized. The UV light intensity was regulated and carefully measured at 400 nm.Since the ex-situ PEC measurements can exclude the effects of mass transfer of organic species in solution, this specific method was adopted to measure the thermodynamic and kinetic properties during PEC degradation of oxalic acid at TiO2 photoanodes. Before the PEC measurement, the photoanode was immersed in a 0.1 M NaNO3 solution with given concentration of oxalic acid at pH 4.0. After the pre-adsorption of organic species reached equilibrium, the photoanode was removed from the adsorption solution, adequately rinsed by 0.1 M NaNO3 solution, and immediately transferred into the PEC cell for PEC measurements under a UV light intensity of 5.0 mW cm−2.
Result and discussion
TiO2 photoanodes with dominated {001} facets
To enable a PEC measurement, a photoanode with immobilised {001} faceted anatase TiO2 needs to be fabricated. However, the synthesis of anatase crystals with exposed {001} facets is challenging because of their high surface energies.31 In 2008, TiO2 nanocrystals with a large percentage of {001} facets have been successfully synthesized through the fluorination techniques.15,25,34 Recently, the immobilization of {001} faceted TiO2 nanoparticles has been reported.31,35 In this work, A001 photoanode with a single layer {001} faceted anatase TiO2 film was fabricated using the method reported by Liu et al.31 The XRD patterns shown in Fig. 1a confirm that the synthesized materials are anatase TiO2. Fig. 1b and c are typical surface and cross-sectional field emission SEM (FESEM) images of A001 photoanode, revealing an approximately 1 μm thick randomly packed TiO2 nanosheets.26 Fig. S3† shows the high resolution transmission electron microscopy (HRTEM) image and selected area electron diffraction (SAED) pattern of the nanosheets, confirming that the nanosheets are formed by 89% exposed {001} facets. The linear sweep voltammograms (LSVs) of the single-layered A001 photoanode under different UV illumination intensities are shown in Fig. 1d. It was found that for all cases investigated, the measured photocurrents increased with the applied potential bias and no saturation can be attained within the applied potential range, which is unsuitable for PEC measurements. This is because the PEC methods11,12,27 used in this work are based on the measurement of the photocurrent/charge originated from the oxidative degradation of oxalic acid. These measurement principles are valid only when the photocurrent profiles are measured at a potential bias where 100% of photogenerated electrons are collected, which can be determined by LSV experiments. In short, under UV illumination, a suitable PEC photoanode should possess a LSV response that initially linearly increased with the applied potential bias (because the electron transport inside the catalyst film is the rate-determining step) and then saturated at more positive potentials (because 100% of photogenerated electrons being collected).10–12,27 One possible reason for the non-saturation behaviour of the single-layered A001 photoanode could be due to the existence of the voids in the large anatase TiO2 nanosheets immobilized on the FTO substrate that allow the electrolyte to directly access to the FTO substrate, leading to a back reaction of photoinjected electrons.36 Under the circumstance, the FTO substrate act as an electrode and the measured current would increase with the increased potential bias.In order to block the electrolyte directly access to the FTO substrate, a double-layered photoanode (DL photoanode) with a dense bottom layer and an A001 top layer was fabricated. Fig. 1e and f show typical surface and cross-sectional FESEM images of DL photoanode. TEM and HRTEM images, and SAED pattern (Fig. 1g) confirm that the top layer is formed by the {001} faceted anatase TiO2 nanosheets.25 The LSVs of DF photoanode with a single layer dense film under different UV illumination intensities confirm that the saturated photocurrents can be attained for all cases investigated (Fig. S4a, ESI†), which are similar to the previous report using the same type photoanode. This demonstrates that a dense film is capable of effectively block the direct electrolyte access to the FTO substrate. The LSVs of DL photoanode under different UV illumination intensities are given in Fig. 1h. The results reveal that for all cases investigated, the photocurrent responses initially increased linearly with the potential bias and saturated when the applied potential bias is more positive than +0.30 V (vs. Ag/AgCl electrode). This means that for the DL photoanode, a 100% electron collection efficiency can be achieved when an applied potential bias is more positive than that of +0.30 V. For better assurance, a +0.40 V potential bias is selected for all subsequent experiments. Under different UV light intensities, the demonstrated linear relationship between light intensities and the saturated photocurrents at the potential bias of +0.4 V (Fig S4b, ESI†) further confirms that the DL photoanode can satisfy the requirements of the PEC measurements in this work if a +0.40 V potential bias is used.
Adsorption properties
The photocatalytic degradation efficiency depends largely on the adsorption properties of organic species on the TiO2 surface.4,6,37 To validate the photoelectrocatalytic performance of the anatase TiO2 (001) surface, an ex situ measurement approach was adopted in this work to qualitatively investigate the surface adsorption properties of {001} faceted photoanode. The oxalic acid is chosen because it is one of the most studies organic species for photocatalytic degradation with great adsorption strength on TiO2 surfaces.9,32,33,38,39 The mineralization of one oxalic molecule required only two electrons and involves the least number of intermediates, which make its overall photocatalytic degradation process simple.Initially, the TiO2 photoanodes were immersed in solutions containing different concentrations of oxalic acid for pre-adsorption. The pre-adsorption time should be long enough to guarantee the pre-adsorption reaches the equilibrium. The relationship between the pre-absorption time and overall net transferred charge (Q) resulted from the mineralization of oxalic acid with a concentration of 0.2 mM was used to determine the per-adsorption time. The Q values are quantified by deducting the changes obtained from integrating photocurrents before and after pre-adsorption (the shaded area in Fig. S5, ESI†).14,27 Fig. S6† confirms that the Q values become steady when the pre-adsorption time exceeds 15 min, indicating equilibrium status has been achieved. For a better assurance, 20 min pre-adsorption time was used in this work. After the pre-adsorption equilibrium was reached, the photoanodes were removed from the adsorption solution and washed by the 0.1 M NaNO3 solution before they were used to perform PEC measurements.
For PEC measurement, the Langmuir adsorption model can be rewritten as:27
 | (1) |
According to eqn (1), a plot of C/Q against C should give a linear relationship and Qmax and K values can thus be determined from the slope and intercept of the plot.
Fig. 2a shows the measured Q–C relationship for oxalic acid with concentrations ranged from 0.1 to 10.0 mM. The corresponding fitting plot for C/Q and C (Fig. 2b) shows a linear (R2 = 0.993) relationship, confirming that the adsorption of oxalic acid on the anatase TiO2 (001) surfaces follows the Langmuir adsorption model within the considered concentration range. The Qmax value obtained from Fig. 2b is 1.61 mC, which is almost identical to that by using the single layered DF electrode (Qmax = 1.60 mC, see Fig. S7, ESI†). Since Qmax represents the maximum saturated adsorption coverage of adsorbents, the similar Qmax values indicate the surface coverage of adsorbed oxalic acid on the on the surface of {001} faceted anatase TiO2 photoanode is close to that on the surface of the DF photoanode. However, the adsorption constant of oxalic acid (K) on the surface of {001} faceted anatase TiO2 photoanode is 2.55 ± 0.96 × 104 M−1, which is ca. 30% larger than that on the surface of the DF photoanode (K = 1.92 ± 0.4 × 104 M−1, Fig. S7, ESI†). Since the DF photoanode is dominated by the exposed {101} facets, the measured adsorption properties support that the reactivity of the anatase (001) surface is higher than that of the anatase (101) surface.21,22
 | ||
| Fig. 2 (a) Plot of Q against the concentration of oxalic acid; (b) Data fitting according to the Langmuir adsorption model shown in eqn (1). | ||
Intrinsic kinetics properties
Understanding intrinsic kinetics is crucially important for catalysts design and performance improvement.3 Theoretically, a heterogeneous photocatalytic reaction should be a first-order reaction if only one type of species adsorbed on the surface.40 However, the experiment data often deviate from a first-order reaction with respect to the concentration of adsorbed species.39 This is because more than one type of adsorbed species may exist on the surface with different atomic configurations and adsorption strength. Previous PEC studies of photocatalytic degradation of oxalic acid by the single layered anatase DF photoanode revealed that the overall photocurrent decay profiles can be well-fitted into a two exponential expression.9,12 The results also confirm that the surface adsorbed complexes can be simultaneously oxidized via two different first-order kinetic processes: one is fast (denoted as f) and another is slow (denoted as s). The two distinct kinetic decay processes represent two different types of adsorbed sites. The slow kinetic process can be attributed to the photocatalytic degradation of the intermediately and weakly bound surface complex, while the fast kinetic process is closely related to the strongly adsorbed surface complex.9,12,31 The overall photocurrent decay profiles can be expressed as:
 | (2) |
The net charge (Q) generated from the photocatalytic oxidation of the adsorbed oxalic acid can therefore be determined by the following equation:
| Q = ∫(Iph − Iblank)dt = ∫(I0phse−kst + I0phfe−kft)dt | (3a) |
| Q = Qs + Qf | (3b) |
In this study, the pre-adsorption of oxalic acid occurs in a 0.1 M NaNO3 solution with the concentration of oxalic acid (C) ranged from 0.1 mM to 10.0 mM at pH 4.0. Fig. 3a shows the photocurrent decay curves from the PEC degradation of the pre-adsorbed oxalic acid on {001} facts dominated DL photoanode. Based on the curve-fitting results using the eqn (2), the values of I0phs, I0phf, ks, and kf, for the PEC degradation of adsorbed oxalic acid on the DL photoanode were calculated (see Table S1, ESI†). The Qs and Qf were then calculated according to eqn (4) (details can be found from eqn S1, ESI†):
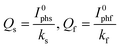 | (4) |
The calculated values for Qs, Qf, and Q based on eqn (3) and (4) are given in Table S2.† The profiles of Qs, Qf, and Q with C are illustrated in Fig. 3b. It can be seen that the main contribution to Q is from Qs, while the Qs–C profile shows a similar trend as that of the Q–C. So, the intermediately and weakly bound surface complexes account for a large portion of the total pre-adsorbed oxalic acid species. On the other hand, the relatively small Qf values indicate that the quantity of strongly bound oxalic acid surface complexes is much less than the intermediate and weakly bound surface complexes.
Plots of I0phs and ks versus Qs for the slow kinetic species are shown in Fig 4a. It can be seen that the I0phs associated with the slow oxidation process are linearly correlated to the net transferred charge Qs, which is determined by the concentration of oxalic acid. The average value of ks is 0.16 s−1, with small variations (±0.01 s−1) over the oxalic acid concentration range investigated. Such small deviations suggest that the ks values are independent of the concentration of oxalic acid. The plots shown in Fig 4b reflect the change of I0phf and kf with the net transferred charge Qf during the fast kinetic process. Similar I0phf–Qf and kf–Qf relationships are found. The average value of kf is 0.75 s−1 with deviations less than 0.02 s−1 over the entire range of considered oxalic acid concentrations. These results are qualitatively agreed with those previously obtained data from the DF photoanode (see Fig. S7, ESI†).9 This means that the adsorption behaviors of oxalic acid on {001} faceted anatase TiO2 surfaces is similar to that of {101} faceted anatase TiO2 surfaces. The above results suggest that the average values of ks and kf obtained from the DL photoanode with exposed {001} facets are 47% and 23% larger than those obtained from the DF photoanode with exposed {101} facets, respectively. The larger rate constants for both the slow and fast kinetic processes obtained from the DL photoanode confirm that the reactivity of the anatase TiO2 (001) surface is higher, as predicted by theoretical studies.21
It should be noted that the presence of the fast and slow kinetic processes suggest the existence of two types of active sites at the surface of the photoanodes,9,12 which could be due to the presence of defects and/or different crystal facets, considering the DL photoanode consists of the mixed {001} and {101} facets.
Conclusions
In summary, a DL photoanode with a {101} facet dominated dense bottom layer and an {001} facet nominated top layer has been purposely fabricated and successfully used for PEC determination of the adsorption constant and the instantaneous intrinsic rate constants of photocatalytic degradation of the adsorbed oxalic acid. The results indicate that the adsorption constant of oxalic acid at a {001} facet dominated surface is 30% larger than that of a {101} facet dominated surface. The results also indicate that the average rate constant values of the slow and fast kinetic processes obtained from a photoanode with exposed {001} facet nominated surface are 47% and 23% larger than those obtained from a photoanode with exposed {101} facet nominated surface. This confirms that an anatase TiO2 photocatalyst with {001} facets dominated surface possess higher photocatalytic activity than a photocatalyst with a {001} facets dominated surface. The method demonstrated in this work could be used to quantitatively investigate the photocatalytic degradation of other organic compounds.Acknowledgements
We thank the Australian Research Council (ARC) and the Natural Science Foundation of China (Grant no. 51372248) for funding.References
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- A. Fujishima and X. T. Zhang, C. R. Chim., 2006, 9, 750–760 CrossRef CAS.
- L. M. Peter, Chem. Rev., 1990, 90, 753–769 CrossRef CAS.
- A. Fujishima, X. T. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS.
- A. L. Linsebigler, G. Q. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- M. A. Henderson, Surf. Sci. Rep., 2011, 66, 185–297 CrossRef CAS.
- A. G. Thomas and K. L. Syres, Chem. Soc. Rev., 2012, 41, 4207–4217 RSC.
- D. L. Jiang, S. Q. Zhang and H. J. Zhao, Environ. Sci. Technol., 2007, 41, 303–308 CrossRef CAS PubMed.
- D. L. Jiang, H. J. Zhao, S. Q. Zhang and R. John, J. Catal., 2004, 223, 212–220 CrossRef CAS.
- D. L. Jiang, H. J. Zhao, S. Q. Zhang and R. John, J. Photochem. Photobiol., A, 2006, 177, 253–260 CrossRef CAS.
- H. Zhao, D. Jiang, S. Zhang, K. Catterall and R. John, Anal. Chem., 2003, 76, 155–160 CrossRef.
- H. Zhao, D. Jiang, S. Zhang and W. Wen, J. Catal., 2007, 250, 102–109 CrossRef CAS.
- D. Jiang, H. Zhao, S. Zhang and R. John, J. Phys. Chem. B, 2003, 107, 12774–12780 CrossRef CAS.
- D. Jiang, H. Zhao, Z. Jia, J. Cao and R. John, J. Photochem. Photobiol., A, 2001, 144, 197–204 CrossRef CAS.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638–641 CrossRef CAS PubMed.
- O. Dulub, M. Batzill, S. Solovev, E. Loginova, A. Alchagirov, T. E. Madey and U. Diebold, Science, 2007, 317, 1052–1056 CrossRef CAS PubMed.
- N. Shibata, A. Goto, S. Y. Choi, T. Mizoguchi, S. D. Findlay, T. Yamamoto and Y. Ikuhara, Science, 2008, 322, 570–573 CrossRef CAS PubMed.
- S. Wendt, P. T. Sprunger, E. Lira, G. K. H. Madsen, Z. S. Li, J. O. Hansen, J. Matthiesen, A. Blekinge-Rasmussen, E. Laegsgaard, B. Hammer and F. Besenbacher, Science, 2008, 320, 1755–1759 CrossRef CAS PubMed.
- Y. Wang, T. Sun, X. L. Liu, H. M. Zhang, P. R. Liu, H. G. Yang, X. D. Yao and H. J. Zhao, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 045304 CrossRef.
- Z. C. Lai, F. Peng, Y. Wang, H. J. Wang, H. Yu, P. R. Liu and H. J. Zhao, J. Mater. Chem., 2012, 22, 23906–23912 RSC.
- X. Q. Gong and A. Selloni, J. Phys. Chem. B, 2005, 109, 19560–19562 CrossRef CAS PubMed.
- A. Vittadini, A. Selloni, F. P. Rotzinger and M. Gratzel, Phys. Rev. Lett., 1998, 81, 2954–2957 CrossRef CAS.
- S. Selcuk and A. Selloni, J. Phys. Chem. C, 2013, 117, 6358–6362 CAS.
- B. Liu and E. S. Aydil, Chem. Commun., 2011, 47, 9507–9509 RSC.
- H. M. Zhang, Y. Wang, P. R. Liu, Y. H. Han, X. D. Yao, J. Zou, H. M. Cheng and H. J. Zhao, ACS Appl. Mater. Interfaces, 2011, 3, 2472–2478 CAS.
- P. R. Liu, Y. Wang, H. M. Zhang, T. C. An, H. G. Yang, Z. Y. Tang, W. P. Cai and H. J. Zhao, Small, 2012, 8, 3664–3673 CrossRef CAS PubMed.
- D. L. Jiang, H. J. Zhao, S. Q. Zhang, R. John and G. D. Will, J. Photochem. Photobiol., A, 2003, 156, 201–206 CrossRef CAS.
- D. Jiang, S. Zhang and H. Zhao, Environ. Sci. Technol., 2006, 41, 303–308 CrossRef.
- S. Zhang, H. Zhao, D. Jiang and R. John, Anal. Chim. Acta, 2004, 514, 89–97 CrossRef CAS.
- M. K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphrybaker, E. Muller, P. Liska, N. Vlachopoulos and M. Gratzel, J. Am. Chem. Soc., 1993, 115, 6382–6390 CrossRef CAS.
- Y. Wang, H. M. Zhang, Y. H. Han, P. R. Liu, X. D. Yao and H. J. Zhao, Chem. Commun., 2011, 47, 2829–2831 RSC.
- T. Berger, A. Rodes and R. Gomez, Phys. Chem. Chem. Phys., 2010, 12, 10503–10511 RSC.
- J. Kulas, I. Rousar, J. Krysa and J. Jirkovsky, J. Appl. Electrochem., 1998, 28, 843–853 CrossRef CAS.
- H. G. Yang, G. Liu, S. Z. Qiao, C. H. Sun, Y. G. Jin, S. C. Smith, J. Zou, H. M. Cheng and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 4078–4083 CrossRef CAS PubMed.
- X. Wang, G. Liu, L. Wang, J. Pan, G. Q. Lu and H.-M. Cheng, J. Mater. Chem., 2011, 21, 869–873 RSC.
- P. J. Cameron, L. M. Peter and S. Hore, J. Phys. Chem. B, 2005, 109, 930–936 CrossRef CAS PubMed.
- U. Diebold, Surf. Sci. Rep., 2003, 48, 53–229 CrossRef CAS.
- I. Ivanova, J. Schneider, H. Gutzmann, J. O. Kliemann, F. Gartner, T. Klassen, D. Bahnemann and C. B. Mendive, Catal. Today, 2013, 209, 84–90 CrossRef CAS.
- C. B. Mendive, T. Bredow, A. Feldhoff, M. A. Blesa and D. Bahnemann, Phys. Chem. Chem. Phys., 2009, 11, 1794–1808 RSC.
- S. R. Morrison, Electrochemistry at Semiconductor and Oxidized Metal Electrodes, Springer, Plenum, New York, 1980 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15336g |
| This journal is © The Royal Society of Chemistry 2015 |



