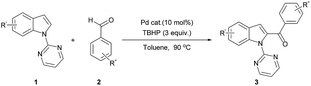
Pd-catalyzed direct C2-acylation and C2,C7-diacylation of indoles: pyrimidine as an easily removable C–H directing group†
Govindharaj
Kumar
and
Govindasamy
Sekar
*
Department of Chemistry, Indian Institute of Technology Madras, Chennai, Tamil Nadu-600 036, India. E-mail: gsekar@iitm.ac.in; Web: http://chem.iitm.ac.in/faculty/sekar/
First published on 13th March 2015
Abstract
Pyrimidine is successfully used as an easily removable C(sp2)–H directing group for the synthesis of 2-acyl indoles and 2,7-diacyl indoles through direct C–H functionalization using a Pd-catalyst from 1-(pyrimidin-2-yl)-1H-indoles and aldehydes. Easy removal of the pyrimidine directing group using EtONa in DMSO provides C2-acyl indoles.
Introduction
Transformation of unactivated C(sp2)–H bonds to C(sp2)–C and C(sp2)-hetero atom bonds is one the most important chemical transformations in synthetic chemistry.1 Transition metal catalysts such as Rh, Ru, Pd, Cu, Fe, etc. play vital roles in C–H bond functionalization reactions. Particularly, Pd compounds occupy a central role as a catalyst for C–H bond functionalization reactions.2 The nature of the directing groups play a crucial role in the activation of unactivated C–H bonds.3 Easily removable directing groups add more advantages to C–H bond functionalization reactions.4 Herein, we report, direct C2-acylation and C2,C7-diacylation of N-pyrimidine substituted indoles to synthesize C2-acyl and C2,C7-diacyl indoles using a Pd-catalyst and aldehyde as an acyl source (Scheme 1). | ||
| Scheme 1 Pd-catalyzed direct C2-acylation and C2,C7-diacylation of indoles using removable pyrimidine directing group. | ||
The direct C–H bond functionalization of indole was started with 1-(pyrimidin-2-yl)-1H-indole 1a. The initial reaction was carried out with 10 mol% of Pd(OAc)2 in chlorobenzene at 90 °C using benzaldehyde 2a as acyl source in the presence of tert-butyl hydrogen peroxide (TBHP) oxidant.5 The pyrimidine group directed the acyl group to the less reactive C2 position of indole ring yielding 43% of expected selective C2-acylated product 3a (Table 1, entry 1). In this reaction, neither C3-acylation nor C7-acylation product was observed. To increase the efficiency of this pyrimidine directed C2-acylation reaction, several Pd-salts were screened and among them PdCl2 provided a maximum of 70% isolated yield of 3a (entry 2).
| Entry | Pd salt | Oxidant | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: 1a (0.5 mmol), 2a (0.75 mmol), and 70% of aq. TBHP. b Isolated yield. c TBHP (5.0 equiv.). | |||||
| 1 | Pd(OAc)2 | TBHP | PhCl | 24 | 43 |
| 2 | PdCl2 | TBHP | PhCl | 12 | 70 |
| 3 | Pd(CH3CN)2Cl2 | TBHP | PhCl | 24 | 47 |
| 4 | Pd(PPh3)2Cl2 | TBHP | PhCl | 24 | 20 |
| 5 | Pd(TFA)2 | TBHP | PhCl | 24 | 55 |
| 6 | Pd2(dba)3 | TBHP | PhCl | 24 | 51 |
| 7 | PdCl2 | DTBP | PhCl | 36 | 40 |
| 8 | PdCl2 | TBPB | PhCl | 36 | 22 |
| 9 | PdCl2 | H2O2 | PhCl | 36 | 20 |
| 10 | PdCl2 | O2 | PhCl | 36 | 14 |
| 11 | PdCl2 | K2S2O8 | PhCl | 36 | 17 |
| 12 | PdCl2 | TBHP | THF | 26 | 21 |
| 13 | PdCl2 | TBHP | DMF | 36 | <5 |
| 14 | PdCl2 | TBHP | Benzene | 15 | 62 |
| 15 | PdCl2 | TBHP | Toluene | 12 | 80 |
| 16 | PdCl2 | TBHP | Dioxane | 24 | 44 |
| 17 | PdCl2 | TBHP | DCE | 24 | 60 |
| 18 | PdCl2 | TBHP | Hexanes | 24 | 26 |
| 19 | PdCl2 | TBHP | Toluene | 12 | 67c |
| 20 | — | TBHP | Toluene | 25 | 00 |
| 21 | PdCl2 | — | Toluene | 26 | 00 |
Then the reaction was screened with several oxidants such as di-tert-butyl peroxide, tert-butyl peroxybenzoate (TBPB), H2O2 etc. and all of them gave inferior result compared with TBHP (entries 6–11). Solvent screening (entries 12–18) was fruitful and toluene provided a maximum of 80% isolated yield of 3a (entry 15). Increasing the quantity of TBHP reduced the efficiency of the C–H activation reaction. And the reaction without Pd-catalyst or oxidant TBHP failed to yield any 2-acylation product (entries 20 and 21).
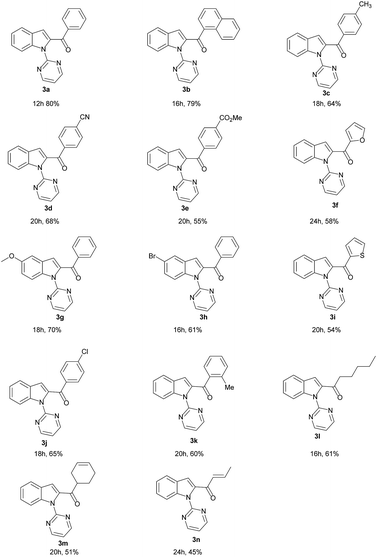
After successful optimizing the reaction conditions for the synthesis of C2-acylated indole, the substrate scope of this synthetic methodology was examined and the results are summarized in Table 2. Both electron-releasing (3c) and electron-withdrawing group (3d and 3e) containing aromatic aldehydes yielded the corresponding 2-acylated products in moderate to good yields. Sterically hindered 1-naphthyl aldehyde also gave C2-acylated product in 79% yield (3b). Heteroaromatic aldehydes such as furan-2-carbaldehyde and thiophene-2-carbaldehyde also provided the corresponding C–H activation product in good yields (3f and 3i). Electron-releasing and electron-withdrawing groups at 5th position of indole also yielded the C2-acylated product in moderate yield range (3g and 3h). Sterically hindered ortho-substituted aldehyde, aliphatic aldehyde, cyclic and α–β unsaturated aldehydes were yielded the expected acylated product in good yield (3k, 3l, 3m and 3n). When a mixture of benzaldehyde and acetaldehyde were used for acylation, only C2-benzoylated indole was selectively obtained. In this reaction condition, acylation took place neither at C2 position nor at C7 position of indole ring.
| a Reaction condition: 1a (0.5 mmol), 2a (0.75 mmol), and 70% of aq. TBHP in toluene (2 mL). All of them are isolated yield. |
|---|
|
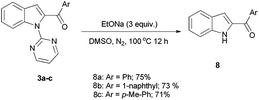
Usage of excess (3 equiv.) aldehyde under same reaction conditions yielded symmetric C2,C7-diacylated product 4 through functionalization of C2 and C7 C–H bonds with good yields. Acylation of two different aldehydes (1.5 equiv. each) one after another aldehyde yielded unsymmetric diacylated products 5. These results clearly shows that in indole molecule, pyrimidine group functionalize both C2 and C7 C–H bonds whereas C2 C–H bond functionalization is more facile than C7 C–H bond of indole moiety (Scheme 2).
When pyrrole and carbazole were used instead of indole molecule, both of them gave mixture of mono and diacylated products as both the C2 and C5 C–H bonds of pyrrole and C2 and C8 C–H bonds of carbazole are identical for C–H functionalization. Using excess of benzaldehyde with N-pyrimidine protected carbazole, the reaction gave C2 and C8-dibenzoylated carbazole (7) with 65% isolated yield (Scheme 3).
The pyrimidine group in acylated indole molecule could be easily removed as shown in Scheme 4.6 Reaction of 3a–c with EtONa in DMSO yielded 2-acylated indole 8a–c. In this C–H acylation, the palladium catalyst will coordinate with pyrimidine nitrogen before activating nearby C–H bond. In the presence of Pd catalyst and TBHP, acyl radical will be generated from aldehyde7 which will be attached to Pd catalyst. Reductive elimination of palladium complex should yield C2-acylated indole. In the presence of excess aldehyde, the mono acylated indole will be acylated at C7 position. This result shows that functionalization at C2 position is easier than functionalization of indole at C7 which is slightly far from indole nitrogen atom.
Conclusions
In conclusion, we have demonstrated an efficient methodology using Pd-catalyst for the synthesis of C2-acylated indole using pyrimidine as directing group. Usage of excess aldehyde provides C2,C7-diacylated indole through double C–H functionalization reaction. Easily removal of pyrimidine directing group using EtONa in DMSO yields C2-acyl indoles.Typical experimental procedure
Phenyl(1-(pyrimidin-2-yl)-1H-indol-2-yl)methanone 3a. Yield 80%; colorless solid; mp = 121–123 °C [122–124 °C];9a Rf = 0.23 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 8
ethyl acetate 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 3408, 2327, 1655, 1570, 1523, 1426, 1277, 748, 717 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.08 (m, 1H), 7.15 (s, 1H), 7.28–7.36 (m, 1H) 7.46 (m, 3H), 7.53–7.63 (m, 1H), 7.73 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 7.6 Hz, 2H), 8.43 (d, J = 8.4, 1H), 8.65 (d, J = 4.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 114.4, 115.5, 117.4, 122.6, 122.9, 126.6, 128.1, 128.4, 129.6, 132.8, 137.3, 138.1, 138.4, 157.4, 158.0, 187.7; HRMS (ESI) calcd for C19H13N3O [M + H]+ 300.1137; found 300.1136.
2); IR (KBr) 3408, 2327, 1655, 1570, 1523, 1426, 1277, 748, 717 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.08 (m, 1H), 7.15 (s, 1H), 7.28–7.36 (m, 1H) 7.46 (m, 3H), 7.53–7.63 (m, 1H), 7.73 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 7.6 Hz, 2H), 8.43 (d, J = 8.4, 1H), 8.65 (d, J = 4.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 114.4, 115.5, 117.4, 122.6, 122.9, 126.6, 128.1, 128.4, 129.6, 132.8, 137.3, 138.1, 138.4, 157.4, 158.0, 187.7; HRMS (ESI) calcd for C19H13N3O [M + H]+ 300.1137; found 300.1136.
Naphthalen-1-yl(1-(pyrimidin-2-yl)-1H-indol-2-yl)methanone 3b. Yield 79%; colorless solid; mp = 136–138 °C [136–138 °C];9a Rf = 0.25 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 8
ethyl acetate 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 3048, 1653, 1573, 1449, 813, 777, 751 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.95 (t, J = 4.8 Hz, 1H), 7.16 (s, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.38 (t, J = 7.6 Hz, 1H), 7.46 (t, J = 8.4 Hz, 1H), 7.51–7.56 (m, 1H), 7.57–7.64 (m, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.76 (d, J = 7.2 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.93 (d, J = 8.4 Hz, 1H), 8.38 (d, J = 8.4 Hz), 8.53 (d, J = 4.8 Hz, 2H), 8.72 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 114.4, 116.3, 117.4, 122.8, 123.0, 124.3, 126.2, 126.5, 126.8, 127.7, 128.1, 128.3, 129.1, 131.2, 132.4, 133.8, 136.2, 138.6, 139.1, 157.5, 158.0, 189.0; HRMS (ESI) calcd for C23H15N3O [M + H]+ 350.1288; found 350.1281.
2); IR (KBr) 3048, 1653, 1573, 1449, 813, 777, 751 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.95 (t, J = 4.8 Hz, 1H), 7.16 (s, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.38 (t, J = 7.6 Hz, 1H), 7.46 (t, J = 8.4 Hz, 1H), 7.51–7.56 (m, 1H), 7.57–7.64 (m, 1H), 7.70 (d, J = 8.0 Hz, 1H), 7.76 (d, J = 7.2 Hz, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.93 (d, J = 8.4 Hz, 1H), 8.38 (d, J = 8.4 Hz), 8.53 (d, J = 4.8 Hz, 2H), 8.72 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 114.4, 116.3, 117.4, 122.8, 123.0, 124.3, 126.2, 126.5, 126.8, 127.7, 128.1, 128.3, 129.1, 131.2, 132.4, 133.8, 136.2, 138.6, 139.1, 157.5, 158.0, 189.0; HRMS (ESI) calcd for C23H15N3O [M + H]+ 350.1288; found 350.1281.
(1-(Pyrimidin-2-yl)-1H-indol-2-yl)(p-tolyl)methanone 3c. Yield 64%; colorless solid; mp = 128–130 °C [121–123 °C];9a Rf = 0.3 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 8
ethyl acetate 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 2924, 2856, 1643, 1570, 1434, 1282, 958, 826, 751 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.41 (s, 3H), 7.04 (t, J = 5.2 Hz, 1H),7.09 (s, 1H), 7.23 (d, J = 8.4 Hz, 1H), 7.38 (t, J = 7.6 Hz, 2H), 7.40–7.46 (m, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 2H), 8.40 (d, J = 8.4 Hz, 1H), 8.62 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.8, 114.4, 115.0, 117.4, 122.5, 122.9, 126.4, 128.2, 129.2, 129.9, 135.6, 137.6, 138.4, 143.6, 157.5, 158.1, 187.5; HRMS (ESI) calcd for C20H15N3O [M + H]+ 314.1281; found 314.1288.
2); IR (KBr) 2924, 2856, 1643, 1570, 1434, 1282, 958, 826, 751 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.41 (s, 3H), 7.04 (t, J = 5.2 Hz, 1H),7.09 (s, 1H), 7.23 (d, J = 8.4 Hz, 1H), 7.38 (t, J = 7.6 Hz, 2H), 7.40–7.46 (m, 1H), 7.69 (d, J = 8.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 2H), 8.40 (d, J = 8.4 Hz, 1H), 8.62 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.8, 114.4, 115.0, 117.4, 122.5, 122.9, 126.4, 128.2, 129.2, 129.9, 135.6, 137.6, 138.4, 143.6, 157.5, 158.1, 187.5; HRMS (ESI) calcd for C20H15N3O [M + H]+ 314.1281; found 314.1288.
4-(1-(Pyrimidin-2-yl)-1H-indole-2-carbonyl)benzonitrile 3d. Yield 68%; white solid; mp = 150–153 °C; Rf = 0.26 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); IR (KBr) 3122, 2226, 1646, 1564, 1434, 1208, 7601 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.07 (t, J = 4.4 Hz, 1H), 7.20 (s, 1H), 7.32 (t, J = 7.6 Hz, 1H), 7.44–7.52 (m, 1H), 7.68–7.76 (m, 3H), 8.01 (d, J = 8.4 Hz, 2H), 8.45 (d, J = 8.4 Hz, 1H), 8.61 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 114.7, 115.8, 116.1, 117.6, 118.2, 122.8, 123.3, 127.2, 128.1, 129.7, 132.3, 136.4, 138.5, 141.7, 157.2, 158.1, 186.0; HRMS (ESI) calcd for C20H12N4O [M + H]+ 325.1089; found 325.1100.
3); IR (KBr) 3122, 2226, 1646, 1564, 1434, 1208, 7601 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.07 (t, J = 4.4 Hz, 1H), 7.20 (s, 1H), 7.32 (t, J = 7.6 Hz, 1H), 7.44–7.52 (m, 1H), 7.68–7.76 (m, 3H), 8.01 (d, J = 8.4 Hz, 2H), 8.45 (d, J = 8.4 Hz, 1H), 8.61 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 114.7, 115.8, 116.1, 117.6, 118.2, 122.8, 123.3, 127.2, 128.1, 129.7, 132.3, 136.4, 138.5, 141.7, 157.2, 158.1, 186.0; HRMS (ESI) calcd for C20H12N4O [M + H]+ 325.1089; found 325.1100.
Methyl-4-(1-(pyrimidin-2-yl)-1H-indole-2-carbonyl)benzoate 3e. Yield 55%; colorless solid; mp = 134–136 °C; Rf = 0.22 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 8
ethyl acetate 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 2938, 1723, 1653, 1569, 1438, 1287, 729 cm−1; 1H NMR (400 MHz, CDCl3) δ 3.94 (s, 3H), 7.04 (t, J = 4.8 Hz, 1H), 7.16 (s, 1H), 7.31 (t, J = 7.2 Hz, 1H), 7.47 (t, J = 7.6 Hz, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.98 (d, J = 8.0 Hz, 2H), 8.08 (d, J = 8.4 Hz, 2H), 8.43 (d, J = 8.4 Hz, 1H), 8.60 (d, J = 4.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 52.6, 114.6, 115.8, 117.5, 122.7, 123.1, 126.9, 128.1, 129.3, 129.7, 133.5, 136.9, 138.4, 141.7, 157.2, 158.1, 166.5, 187.0; HRMS (ESI) calcd for C21H16N3O3 [M + H]+ 358.1192; found 358.1184.
2); IR (KBr) 2938, 1723, 1653, 1569, 1438, 1287, 729 cm−1; 1H NMR (400 MHz, CDCl3) δ 3.94 (s, 3H), 7.04 (t, J = 4.8 Hz, 1H), 7.16 (s, 1H), 7.31 (t, J = 7.2 Hz, 1H), 7.47 (t, J = 7.6 Hz, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.98 (d, J = 8.0 Hz, 2H), 8.08 (d, J = 8.4 Hz, 2H), 8.43 (d, J = 8.4 Hz, 1H), 8.60 (d, J = 4.4 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 52.6, 114.6, 115.8, 117.5, 122.7, 123.1, 126.9, 128.1, 129.3, 129.7, 133.5, 136.9, 138.4, 141.7, 157.2, 158.1, 166.5, 187.0; HRMS (ESI) calcd for C21H16N3O3 [M + H]+ 358.1192; found 358.1184.
Furan-2-yl(1-(pyrimidin-2-yl)-1H-indol-2-yl)methanone 3f. Yield 58%; light yellow solid; mp = 120–122 °C [122–124 °C];9a Rf = 0.26 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); IR (KBr) 2926, 1640, 1568, 1468, 1292, 827, 793, 750 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.47 (d, J = 1.6 Hz, 1H), δ 7.03 (t, J = 4.8 Hz, 1H), δ 7.16–7.25 (m, 2H), δ 7.29 (s, 1H), δ 7.36 (t, J = 7.6 Hz, 1H), δ 7.55 (s, 1H), 7.65 (d, J = 7.6 Hz, 1H), δ 8.27 (d, J = 8.4 Hz, 1H), δ 8.61 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 112.4, 114.2, 115.4, 117.6, 119.5, 122.7, 122.9, 126.8, 128.0, 136.2, 138.7, 147.0, 152.7, 157.5, 158.2, 174.4; HRMS (ESI) calcd for C17H12N3O2 [M + H]+ 290.0930; found 290.0933.
3); IR (KBr) 2926, 1640, 1568, 1468, 1292, 827, 793, 750 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.47 (d, J = 1.6 Hz, 1H), δ 7.03 (t, J = 4.8 Hz, 1H), δ 7.16–7.25 (m, 2H), δ 7.29 (s, 1H), δ 7.36 (t, J = 7.6 Hz, 1H), δ 7.55 (s, 1H), 7.65 (d, J = 7.6 Hz, 1H), δ 8.27 (d, J = 8.4 Hz, 1H), δ 8.61 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 112.4, 114.2, 115.4, 117.6, 119.5, 122.7, 122.9, 126.8, 128.0, 136.2, 138.7, 147.0, 152.7, 157.5, 158.2, 174.4; HRMS (ESI) calcd for C17H12N3O2 [M + H]+ 290.0930; found 290.0933.
(5-Methoxy-1-(pyrimidin-2-yl)-1H-indol-2-yl)(phenyl)methanone 3g. Yield 70%; colorless solid; mp = 129–131 °C [134–135 °C];9a Rf = 0.20 (hexanes–ethyl acetate 8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 2987, 2361, 1657, 1607, 1440, 1234, 804, 706 cm−1; 1H NMR (400 MHz, CDCl3) δ 3.88 (s, 3H), 7.02 (t, J = 4.8 Hz, 1H), 7.05 (d, J = 0.4 Hz, 1H), 7.08 (dd, J = 9.2, 2.4 Hz, 1H), 7.12 (d, J = 2.4 Hz, 1H), 7.39–7.46 (m, 1H), 7.50–7.56 (m, 1H), 7.92–7.98 (m, 2H), 8.34 (d, J = 9.2 Hz, 1H), 8.59 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 55.8, 103.6, 115.1, 115.5, 116.6, 117.3, 128.4, 128.8, 129.6, 132.8, 133.3, 137.7, 138.2, 156.1, 157.3, 158.0, 187.8; HRMS (ESI) calcd for C20H16N3O2 [M + H]+ 330.1243; found 330.1230.
2); IR (KBr) 2987, 2361, 1657, 1607, 1440, 1234, 804, 706 cm−1; 1H NMR (400 MHz, CDCl3) δ 3.88 (s, 3H), 7.02 (t, J = 4.8 Hz, 1H), 7.05 (d, J = 0.4 Hz, 1H), 7.08 (dd, J = 9.2, 2.4 Hz, 1H), 7.12 (d, J = 2.4 Hz, 1H), 7.39–7.46 (m, 1H), 7.50–7.56 (m, 1H), 7.92–7.98 (m, 2H), 8.34 (d, J = 9.2 Hz, 1H), 8.59 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 55.8, 103.6, 115.1, 115.5, 116.6, 117.3, 128.4, 128.8, 129.6, 132.8, 133.3, 137.7, 138.2, 156.1, 157.3, 158.0, 187.8; HRMS (ESI) calcd for C20H16N3O2 [M + H]+ 330.1243; found 330.1230.
(5-Bromo-1-(pyrimidin-2-yl)-1H-indol-2-yl)(phenyl)methanone 3h. Yield 61%; light yellow solid; mp = 116–118 °C [120–122 °C];9a Rf = 0.25 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate); IR (KBr) 2922, 1667, 1572, 1443, 800, 713 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.02 (s, 1H), 7.04 (t, J = 8.4 Hz, 1H), 7.43 (t, J = 7.6 Hz, 2H), 7.50 (dd, J = 9.2, 2.0 Hz, 1H), 7.52–757 (m, 1H), 7.81 (d, J = 1.6 Hz, 1H), 7.91–7.98 (m, 2H), 8.32 (d, J = 9.2 Hz, 1H), 8.59 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 113.7, 116.0, 116.1, 117.7, 124.8, 128.5, 129.2, 129.5, 129.8, 133.0, 136.7, 137.7, 138.1, 156.9, 158.1, 187.5; HRMS (ESI) calcd for C19H13N3OBr [M + H]+ 378.0242; found 378.0229.
ethyl acetate); IR (KBr) 2922, 1667, 1572, 1443, 800, 713 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.02 (s, 1H), 7.04 (t, J = 8.4 Hz, 1H), 7.43 (t, J = 7.6 Hz, 2H), 7.50 (dd, J = 9.2, 2.0 Hz, 1H), 7.52–757 (m, 1H), 7.81 (d, J = 1.6 Hz, 1H), 7.91–7.98 (m, 2H), 8.32 (d, J = 9.2 Hz, 1H), 8.59 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 113.7, 116.0, 116.1, 117.7, 124.8, 128.5, 129.2, 129.5, 129.8, 133.0, 136.7, 137.7, 138.1, 156.9, 158.1, 187.5; HRMS (ESI) calcd for C19H13N3OBr [M + H]+ 378.0242; found 378.0229.
(1-(Pyrimidin-2-yl)-1H-indol-2-yl)(thiophen-2-yl)methanone 3i. Yield 54%; colorless solid; mp = 144–146 °C [136–138 °C];9a Rf = 0.20 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 8
ethyl acetate 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 3071, 1624, 1570, 1521, 1425, 742 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.00–7.04 (m, 1H), 7.05–7.09 (m, 1H), 7.17–7.25 (m, 2H), 7.33–7.39 (m, 1H), 7.60–7.66 (m, 2H), 7.75 (dd, J = 4, 1.2 Hz, 1H), 8.29 (dd, J = 8.4, 0.4 Hz, 1H), 8.61 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 113.7, 116.2, 116.3, 117.8, 124.9, 128.9, 129.4, 129.8, 130.9, 136.2, 136.7, 137.7, 139.5, 157.0, 158.2, 186.4; HRMS (ESI) calcd for C17H12N3O [M + H]+ 306.0701; found 306.0711.
2); IR (KBr) 3071, 1624, 1570, 1521, 1425, 742 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.00–7.04 (m, 1H), 7.05–7.09 (m, 1H), 7.17–7.25 (m, 2H), 7.33–7.39 (m, 1H), 7.60–7.66 (m, 2H), 7.75 (dd, J = 4, 1.2 Hz, 1H), 8.29 (dd, J = 8.4, 0.4 Hz, 1H), 8.61 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 113.7, 116.2, 116.3, 117.8, 124.9, 128.9, 129.4, 129.8, 130.9, 136.2, 136.7, 137.7, 139.5, 157.0, 158.2, 186.4; HRMS (ESI) calcd for C17H12N3O [M + H]+ 306.0701; found 306.0711.
(4-Chlorophenyl)(1-(pyrimidin-2-yl)-1H-indol-2-yl)methanone 3j. Yield 54%; colorless solid; mp = 144–146 °C [136–138 °C];9a Rf = 0.20 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 8
ethyl acetate 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 3072, 1623, 1569, 1520, 1426, 741 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.00–7.04 (m, 1H), 7.05–7.08 (m, 1H), 7.17–7.24 (m, 2H), 7.34–7.39 (m, 1H), 7.60–7.65 (m, 2H), 7.75 (dd, J = 4, 1.2 Hz, 1H), 8.29 (dd, J = 8.4, 0.4 Hz, 1H), 8.61 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 113.7, 116.2, 116.3, 117.8, 124.9, 128.9, 129.4, 129.8, 130.9, 136.2, 136.7, 137.7, 139.5, 157.0, 158.2, 186.4; HRMS (ESI) calcd for C17H12N3O [M + H]+ 306.0701; found 306.0711.
2); IR (KBr) 3072, 1623, 1569, 1520, 1426, 741 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.00–7.04 (m, 1H), 7.05–7.08 (m, 1H), 7.17–7.24 (m, 2H), 7.34–7.39 (m, 1H), 7.60–7.65 (m, 2H), 7.75 (dd, J = 4, 1.2 Hz, 1H), 8.29 (dd, J = 8.4, 0.4 Hz, 1H), 8.61 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 113.7, 116.2, 116.3, 117.8, 124.9, 128.9, 129.4, 129.8, 130.9, 136.2, 136.7, 137.7, 139.5, 157.0, 158.2, 186.4; HRMS (ESI) calcd for C17H12N3O [M + H]+ 306.0701; found 306.0711.
(1-(Pyrimidin-2-yl)-1H-indol-2-yl)(o-tolyl)methanone 3k. Yield 60%; colorless solid; mp = 124–126 °C; Rf = 0.20 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 8
ethyl acetate 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 3050, 1653, 1540, 1520, 1450, 756 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.59 (s, 3H), 7.11 (s, 2H), 7.18 (t, J = 7.6 Hz, 1H), 7.24–7.33 (m, 2H), 7.37 (td, J = 6, 1.2 Hz, 1H), 7.43–7.49 (m, 1H), 7.56–7.61 (m, 1H), 7.70 (d, J = 8.0 Hz, 1H), 8.32 (d, J = 8.4 Hz, 1H), 8.69 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 20.5, 114.1, 116.3, 117.7, 122.8, 122.9, 125.2, 126.9, 128.0, 130.1, 131.2, 131.3, 138.3, 138.8, 138.9, 157.6, 158.1, 189.4; HRMS (ESI) calcd for C20H15N3O [M + H]+ 314.1284; found 314.1288.
2); IR (KBr) 3050, 1653, 1540, 1520, 1450, 756 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.59 (s, 3H), 7.11 (s, 2H), 7.18 (t, J = 7.6 Hz, 1H), 7.24–7.33 (m, 2H), 7.37 (td, J = 6, 1.2 Hz, 1H), 7.43–7.49 (m, 1H), 7.56–7.61 (m, 1H), 7.70 (d, J = 8.0 Hz, 1H), 8.32 (d, J = 8.4 Hz, 1H), 8.69 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 20.5, 114.1, 116.3, 117.7, 122.8, 122.9, 125.2, 126.9, 128.0, 130.1, 131.2, 131.3, 138.3, 138.8, 138.9, 157.6, 158.1, 189.4; HRMS (ESI) calcd for C20H15N3O [M + H]+ 314.1284; found 314.1288.
1-(1-(Pyrimidin-2-yl)-1H-indol-2-yl)hexan-1-one 3l. Yield 61%; liquid; Rf = 0.34 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 8
ethyl acetate 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 3069, 1640, 1558, 1510, 1458, 860, 790, 756 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.82 (t, J = 7.2 Hz, 3H), 1.23–1.36 (m, 4H), 1.63–1.74 (m, 2H), 2.84 (t, J = 7.2 Hz, 2H), 7.12 (t, J = 4.8 Hz, 1H), 7.14–7.19 (m, 1H), 7.21 (s, 1H), 7.27–7.33 (m, 1H), 7.59–7.62 (m, 1H), 7.89–7.92 (m, 1H), 8.68 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 14.1, 22.6, 24.5, 31.6, 40.6, 113.3, 113.5, 118.3, 122.7, 122.8, 126.8, 127.6, 138.1, 139.3, 158.0, 158.1, 158.4, 194.1; HRMS (ESI) calcd for C18H19N3O [M + H]+ 294.1597; found 294.1601.
2); IR (KBr) 3069, 1640, 1558, 1510, 1458, 860, 790, 756 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.82 (t, J = 7.2 Hz, 3H), 1.23–1.36 (m, 4H), 1.63–1.74 (m, 2H), 2.84 (t, J = 7.2 Hz, 2H), 7.12 (t, J = 4.8 Hz, 1H), 7.14–7.19 (m, 1H), 7.21 (s, 1H), 7.27–7.33 (m, 1H), 7.59–7.62 (m, 1H), 7.89–7.92 (m, 1H), 8.68 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 14.1, 22.6, 24.5, 31.6, 40.6, 113.3, 113.5, 118.3, 122.7, 122.8, 126.8, 127.6, 138.1, 139.3, 158.0, 158.1, 158.4, 194.1; HRMS (ESI) calcd for C18H19N3O [M + H]+ 294.1597; found 294.1601.
Cyclohex-3-en-1-yl(1-(pyrimidin-2-yl)-1H-indol-2-yl)methanone 3m. Yield 51%; colourless solid; mp = 122–124 °C Rf = 0.23 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 8
ethyl acetate 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); IR (KBr) 3040, 1655, 1556, 1515, 1470, 1100, 840, 800, 745 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.73–1.87 (m, 1H), 2.08–2.23 (m, 3H), 2.27–2.37 (m, 1H), 2.40–2.52 (m, 1H), 3.26–3.35 (m, 1H), 5.80 (s, 2H), 7.21 (t, J = 4.8 Hz, 1H), 7.28 (t, J = 7.6 Hz, 2H), 7.42 (td, J = 7.2, 1.2 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.80 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 25.0, 25.9, 28.0, 44.8, 113.0, 113.8, 118.1, 122.7, 122.8, 125.9, 126.7, 126.7, 127.9, 137.7, 139.1, 158.0, 158.3, 197.3; HRMS (ESI) calcd for C19H17N3O [M + H]+ 304.1450; found 304.1446.
2); IR (KBr) 3040, 1655, 1556, 1515, 1470, 1100, 840, 800, 745 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.73–1.87 (m, 1H), 2.08–2.23 (m, 3H), 2.27–2.37 (m, 1H), 2.40–2.52 (m, 1H), 3.26–3.35 (m, 1H), 5.80 (s, 2H), 7.21 (t, J = 4.8 Hz, 1H), 7.28 (t, J = 7.6 Hz, 2H), 7.42 (td, J = 7.2, 1.2 Hz, 1H), 7.73 (d, J = 8.0 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 8.80 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 25.0, 25.9, 28.0, 44.8, 113.0, 113.8, 118.1, 122.7, 122.8, 125.9, 126.7, 126.7, 127.9, 137.7, 139.1, 158.0, 158.3, 197.3; HRMS (ESI) calcd for C19H17N3O [M + H]+ 304.1450; found 304.1446.
(E)-1-(1-(Pyrimidin-2-yl)-1H-indol-2-yl)but-2-en-1-one 3n. Yield 45%; light yellow solid; mp = 123–125 °C; Rf = 0.30 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 6
ethyl acetate 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); IR (KBr) 3100, 3020, 1665, 1585, 1520, 1426, 1010, 860, 756 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.00 (d, J = 6.8 Hz, 3H), 6.71 (d, J = 15.6 Hz, 1H), 6.97–7.09 (m, 1H), 7.21 (t, J = 4.8 Hz, 1H), 7.28 (t, J = 8.0 Hz, 2H), 7.42 (t, J = 7.6 Hz, 1H), 7.73 (d, J = 7.6 Hz, 1H), 8.15 (d, J = 8.4 Hz, 1H), 8.79 (d, J = 4.8 Hz, 2H); 13C NMR 18.6, 113.6, 114.3, 118.1, 122.7, 126.7, 127.8, 130.3, 138.0, 139.2, 144.4, 157.9, 158.3, 184.1; HRMS (ESI) calcd for C16H13N3O [M + H]+ 264.1137; found 264.1129.
4); IR (KBr) 3100, 3020, 1665, 1585, 1520, 1426, 1010, 860, 756 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.00 (d, J = 6.8 Hz, 3H), 6.71 (d, J = 15.6 Hz, 1H), 6.97–7.09 (m, 1H), 7.21 (t, J = 4.8 Hz, 1H), 7.28 (t, J = 8.0 Hz, 2H), 7.42 (t, J = 7.6 Hz, 1H), 7.73 (d, J = 7.6 Hz, 1H), 8.15 (d, J = 8.4 Hz, 1H), 8.79 (d, J = 4.8 Hz, 2H); 13C NMR 18.6, 113.6, 114.3, 118.1, 122.7, 126.7, 127.8, 130.3, 138.0, 139.2, 144.4, 157.9, 158.3, 184.1; HRMS (ESI) calcd for C16H13N3O [M + H]+ 264.1137; found 264.1129.
(1-(Pyrimidin-2-yl)-1H-indole-2,7-diyl)bis(phenylmethanone) 4a. Yield 72%; light yellow solid; mp = 160–161 °C; Rf = 0.25 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); IR (KBr) 3042, 1645, 1562, 1443, 863, 717 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.96 (t, J = 4.8 Hz, 1H), 7.19 (s, 1H), 7.28 (t, J = 7.6 Hz, 1H), 7.37–7.40 (m, 2H), 7.41–7.44 (m, 2H), 7.45–7.48 (m, 2H), 7.49–7.53 (m, 1H), 7.54–7.59 (m, 1H), 7.78–7.82 (m, 2H), 7.86 (dd, J = 8.0, 1.2 Hz, 2H), 7.98 (dd, J = 8.0, 1.2 Hz, 2H), 8.34 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 114.8, 118.9, 121.4, 125.6, 125.9, 127.8, 128.3, 128.5, 128.8, 129.9, 130.1, 132.8, 133.0, 135.2, 137.3, 137.7, 137.7, 157.6, 157.9, 187.1, 195.4; HRMS (ESI) calcd for C26H18N3O2 [M + H]+ 404.1399; found 404.1392.
3); IR (KBr) 3042, 1645, 1562, 1443, 863, 717 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.96 (t, J = 4.8 Hz, 1H), 7.19 (s, 1H), 7.28 (t, J = 7.6 Hz, 1H), 7.37–7.40 (m, 2H), 7.41–7.44 (m, 2H), 7.45–7.48 (m, 2H), 7.49–7.53 (m, 1H), 7.54–7.59 (m, 1H), 7.78–7.82 (m, 2H), 7.86 (dd, J = 8.0, 1.2 Hz, 2H), 7.98 (dd, J = 8.0, 1.2 Hz, 2H), 8.34 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 114.8, 118.9, 121.4, 125.6, 125.9, 127.8, 128.3, 128.5, 128.8, 129.9, 130.1, 132.8, 133.0, 135.2, 137.3, 137.7, 137.7, 157.6, 157.9, 187.1, 195.4; HRMS (ESI) calcd for C26H18N3O2 [M + H]+ 404.1399; found 404.1392.
(1-(Pyrimidin-2-yl)-1H-indole-2,7-diyl)bis(p-tolylmethanone) 4b. Yield 52%; white solid mp = 179–180 °C Rf = 0.26 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); IR (KBr) 3036, 1650, 1602, 1427, 1276, 1214, 749, 700 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 2.36 (s, 3H), 2.36 (s, 3H), 6.91 (t, J = 4.8 Hz, 1H), 7.09 (s, 1H), 7.13 (d, J = 8.0 Hz, 2H), 7.17–7.22 (m, 3H), 7.31 (dd, J = 7.2, 1.2 Hz, 1H), 7.63 (d, J = 8.4 Hz, 2H), 7.77 (dd, J = 8.0, 1.2 Hz, 1H), 7.83 (d, J = 8.4 Hz, 2H), 8.29 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.8, 114.5, 118.9, 121.3, 125.4, 126.1, 127.6, 128.8, 129.1, 129.2, 130.2, 130.4, 135.1, 135.2, 137.4, 143.7, 143.9, 157.7, 157.9, 186.9, 195.3; HRMS (ESI) calcd for C28H22N3O2 [M + H]+ 432.1712; found 432.1709.
3); IR (KBr) 3036, 1650, 1602, 1427, 1276, 1214, 749, 700 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 2.36 (s, 3H), 2.36 (s, 3H), 6.91 (t, J = 4.8 Hz, 1H), 7.09 (s, 1H), 7.13 (d, J = 8.0 Hz, 2H), 7.17–7.22 (m, 3H), 7.31 (dd, J = 7.2, 1.2 Hz, 1H), 7.63 (d, J = 8.4 Hz, 2H), 7.77 (dd, J = 8.0, 1.2 Hz, 1H), 7.83 (d, J = 8.4 Hz, 2H), 8.29 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.8, 114.5, 118.9, 121.3, 125.4, 126.1, 127.6, 128.8, 129.1, 129.2, 130.2, 130.4, 135.1, 135.2, 137.4, 143.7, 143.9, 157.7, 157.9, 186.9, 195.3; HRMS (ESI) calcd for C28H22N3O2 [M + H]+ 432.1712; found 432.1709.
(1-(Pyrimidin-2-yl)-1H-indole-2,7-diyl)bis(cyclohexylmethanone) 4c. Yield 50%; white solid; mp = 163–165 °C Rf = 0.26 (hexanes–ethyl acetate 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); IR (KBr) 3058, 1656, 1573, 1428, 1350, 1214, 1150, 835, 720 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.3–1.24 (m, 3H), 1.25–1.45 (m, 4H), 1.47–1.60 (m, 4H), 1.69–1.90 (m, 5H), 1.96–2.12 (m, 5H), 2.68 (tt, J = 11.6, 3.6 Hz, 1H), 3.08 (tt, J = 11.2, 3.6 Hz, 1H), 7.19 (t, J = 4.8 Hz, 1H), 7.36–7.47 (m, 2H), 7.85–7.92 (m, 1H), 8.69 (d, J = 8.4, Hz) 8.74 (d, J = 4.8, Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 26.0, 26.1, 26.2, 29.0, 29.1, 50.3, 51.6, 116.0, 118.0, 121.0, 121.3, 124.2, 125.5, 126.7, 135.9, 140.6, 156.9, 158.1, 201.9, 202.2; HRMS (ESI) calcd for C18H19N3O [M + H]+ 294.1597; found 294.160.
3); IR (KBr) 3058, 1656, 1573, 1428, 1350, 1214, 1150, 835, 720 cm−1; 1H NMR (400 MHz, CDCl3) δ 1.3–1.24 (m, 3H), 1.25–1.45 (m, 4H), 1.47–1.60 (m, 4H), 1.69–1.90 (m, 5H), 1.96–2.12 (m, 5H), 2.68 (tt, J = 11.6, 3.6 Hz, 1H), 3.08 (tt, J = 11.2, 3.6 Hz, 1H), 7.19 (t, J = 4.8 Hz, 1H), 7.36–7.47 (m, 2H), 7.85–7.92 (m, 1H), 8.69 (d, J = 8.4, Hz) 8.74 (d, J = 4.8, Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 26.0, 26.1, 26.2, 29.0, 29.1, 50.3, 51.6, 116.0, 118.0, 121.0, 121.3, 124.2, 125.5, 126.7, 135.9, 140.6, 156.9, 158.1, 201.9, 202.2; HRMS (ESI) calcd for C18H19N3O [M + H]+ 294.1597; found 294.160.
4-(2-Benzoyl-1-(pyrimidin-2-yl)-1H-indole-7-carbonyl)benzonitrile 5a. Yield 64%; white solid; mp = 190–191 °C Rf = 0.26 (hexanes–ethyl acetate 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); IR (KBr) 2924, 2231, 1656, 1573, 1428, 1255, 1214, 718 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.00 (t, J = 4.8 Hz, 1H), 7.21 (s, 1H), 7.29–7.37 (m, 2H), 7.45–7.51 (m, 2H), 7.60 (tt, J = 7.6, 1.2 Hz, 1H), 7.70–7.76 (m, 2H), 7.89–7.95 (m, 3H), 7.96–8.01 (m, 2H), 8.35 (t, J = 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 114.8, 116.1, 118.2, 119.0, 121.7, 125.0, 126.3, 127.5, 128.6, 129.2, 129.9, 130.3, 132.3, 134.8, 137.6, 137.8, 141.1, 157.3, 157.8, 187.2, 193.6; HRMS (ESI) calcd for C27H17N4O2 [M + H]+ 429.1352; found 429.1355.
3); IR (KBr) 2924, 2231, 1656, 1573, 1428, 1255, 1214, 718 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.00 (t, J = 4.8 Hz, 1H), 7.21 (s, 1H), 7.29–7.37 (m, 2H), 7.45–7.51 (m, 2H), 7.60 (tt, J = 7.6, 1.2 Hz, 1H), 7.70–7.76 (m, 2H), 7.89–7.95 (m, 3H), 7.96–8.01 (m, 2H), 8.35 (t, J = 4.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 114.8, 116.1, 118.2, 119.0, 121.7, 125.0, 126.3, 127.5, 128.6, 129.2, 129.9, 130.3, 132.3, 134.8, 137.6, 137.8, 141.1, 157.3, 157.8, 187.2, 193.6; HRMS (ESI) calcd for C27H17N4O2 [M + H]+ 429.1352; found 429.1355.
(2-Benzoyl-1-(pyrimidin-2-yl)-1H-indol-7-yl)(p-tolyl)methanone 5b. Yield 68%; light yellow solid; mp = 179–180 °C Rf = 0.26 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); IR (KBr) 2925, 1649, 1570, 1422, 1262, 747, 716 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 6.92 (t, J = 4.8 Hz, 1H), 7.12 (s, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.17–7.23 (m, 1H), 7.32 (dd, J = 7.2, 1.2 Hz, 1H), 7.39 (t, J = 7.6 Hz, 2H), 7.51 (t, J = 7.6 Hz, 1H), 7.63 (d, J = 8.0 Hz, 2H), 7.78 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 6.8 Hz, 2H), 8.30 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.8, 114.9, 119.0, 121.4, 125.5, 126.1, 127.8, 128.4, 128.5, 128.8, 129.1, 130.0, 130.2, 130.4, 133.1, 135.1, 135.3, 137.2, 137.8, 143.7, 157.7, 158.0, 187.1, 195.2; HRMS (ESI) calcd for C27H20N3O2 [M + H]+ 418.1556; found 418.1572.
3); IR (KBr) 2925, 1649, 1570, 1422, 1262, 747, 716 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 6.92 (t, J = 4.8 Hz, 1H), 7.12 (s, 1H), 7.14 (d, J = 8.0 Hz, 2H), 7.17–7.23 (m, 1H), 7.32 (dd, J = 7.2, 1.2 Hz, 1H), 7.39 (t, J = 7.6 Hz, 2H), 7.51 (t, J = 7.6 Hz, 1H), 7.63 (d, J = 8.0 Hz, 2H), 7.78 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 6.8 Hz, 2H), 8.30 (d, J = 4.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 21.8, 114.9, 119.0, 121.4, 125.5, 126.1, 127.8, 128.4, 128.5, 128.8, 129.1, 130.0, 130.2, 130.4, 133.1, 135.1, 135.3, 137.2, 137.8, 143.7, 157.7, 158.0, 187.1, 195.2; HRMS (ESI) calcd for C27H20N3O2 [M + H]+ 418.1556; found 418.1572.
(9-(Pyrimidin-2-yl)-9H-carbazole-1,8-diyl)bis(phenylmethanone) 7. Yield 62%; light yellow solid; mp = 160–162 °C; Rf = 0.28 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 7
ethyl acetate 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); IR (KBr) 3090, 1670, 1530, 1510, 1434, 1208, 854, 800, 760 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.72 (t, J = 5.2 Hz, 1H), 7.38 (t, J = 7.6 Hz, 4H), 7.42–7.49 (m, 4H), 7.50–7.52 (m, 2H), 7.83 (d, J = 7.2 Hz, 4H), 7.96 (d, J = 4.8 Hz, 2H), 8.27 (d, J = 7.6 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 118.0, 122.1, 122.2, 126.7, 127.6, 128.0, 128.3, 129.9, 134.5, 136.9, 137.9, 156.6, 156.8, 194.7; HRMS (ESI) calcd for C30H19N3O2 [M + H]+ 454.1556; found 454.1551.
3); IR (KBr) 3090, 1670, 1530, 1510, 1434, 1208, 854, 800, 760 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.72 (t, J = 5.2 Hz, 1H), 7.38 (t, J = 7.6 Hz, 4H), 7.42–7.49 (m, 4H), 7.50–7.52 (m, 2H), 7.83 (d, J = 7.2 Hz, 4H), 7.96 (d, J = 4.8 Hz, 2H), 8.27 (d, J = 7.6 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 118.0, 122.1, 122.2, 126.7, 127.6, 128.0, 128.3, 129.9, 134.5, 136.9, 137.9, 156.6, 156.8, 194.7; HRMS (ESI) calcd for C30H19N3O2 [M + H]+ 454.1556; found 454.1551.
(1H-Indol-2-yl)(phenyl)methanone 8a. Yield 75%; white solid; mp = 145–146 °C [149–150 °C];9b Rf = 0.26 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 9
ethyl acetate 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); IR (KBr) 3354, 1620, 1517, 1260, 1125, 736, 680 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.14–7.21 (m, 2H), 7.35–7.42 (m, 1H), 7.47–7.59 (m, 3H), 7.60–7.67 (m, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.98–8.1 (m, 2H), 9.56 (bs, 1H); 13C NMR (100 MHz, CDCl3) δ 112.4, 113.0, 121.2, 123.4, 126.7, 127.9, 128.6, 129.4, 132.5, 134.5, 137.7, 138.2, 187.4; HRMS (ESI) calcd for C15H12NO [M + H]+ 222.0919; found 222.0917.
1); IR (KBr) 3354, 1620, 1517, 1260, 1125, 736, 680 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.14–7.21 (m, 2H), 7.35–7.42 (m, 1H), 7.47–7.59 (m, 3H), 7.60–7.67 (m, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.98–8.1 (m, 2H), 9.56 (bs, 1H); 13C NMR (100 MHz, CDCl3) δ 112.4, 113.0, 121.2, 123.4, 126.7, 127.9, 128.6, 129.4, 132.5, 134.5, 137.7, 138.2, 187.4; HRMS (ESI) calcd for C15H12NO [M + H]+ 222.0919; found 222.0917.
(1H-Indol-2-yl)(naphthalen-1-yl)methanone 8b. Yield 73%; light yellow solid; mp = 144–145 °C [150–151 °C];9c Rf = 0.26 (hexanes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 9
ethyl acetate 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); IR (KBr) 3327, 1614, 1520, 783, 746 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.98 (dd, J = 2.0, 0.8 Hz, 1H), 7.13–7.19 (m, 1H), 7.35–7.42 (m, 1H), 7.52 (dd, J = 8.4, 0.8 Hz, 1H), 7.54–7.60 (m, 3H), 7.66 (dd, J = 8.0, 0.8 Hz, 1H), 7.90 (dd, J = 7.2, 1.2 Hz, 1H) 7.92–7.98 (m, 1H), 8.05 (d, J = 8.4 Hz, 1H), 8.28–8.34 (m, 1H), 9.75 (bs, 1H); 13C NMR (100 MHz, CDCl3) δ 112.5, 113.9, 121.2, 123.5, 124.5, 125.7, 126.7, 126.9, 127.5, 127.7, 127.9, 128.5, 131.0, 131.7, 134.0, 135.8, 136.2, 138.2, 189.0; HRMS (ESI) calcd for C19H14NO [M + H]+ 272.1075; found 272.1077.
1); IR (KBr) 3327, 1614, 1520, 783, 746 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.98 (dd, J = 2.0, 0.8 Hz, 1H), 7.13–7.19 (m, 1H), 7.35–7.42 (m, 1H), 7.52 (dd, J = 8.4, 0.8 Hz, 1H), 7.54–7.60 (m, 3H), 7.66 (dd, J = 8.0, 0.8 Hz, 1H), 7.90 (dd, J = 7.2, 1.2 Hz, 1H) 7.92–7.98 (m, 1H), 8.05 (d, J = 8.4 Hz, 1H), 8.28–8.34 (m, 1H), 9.75 (bs, 1H); 13C NMR (100 MHz, CDCl3) δ 112.5, 113.9, 121.2, 123.5, 124.5, 125.7, 126.7, 126.9, 127.5, 127.7, 127.9, 128.5, 131.0, 131.7, 134.0, 135.8, 136.2, 138.2, 189.0; HRMS (ESI) calcd for C19H14NO [M + H]+ 272.1075; found 272.1077.
(1H-Indol-2-yl)(p-tolyl)methanone 8c. Yield 71%; light yellow solid; mp = 174–176 °C [187–188 °C];9d Rf = 0.26 (hexanes–ethyl acetate 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); IR (KBr) 3350, 1616, 1515, 1261, 825, 750 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 7.14–7.20 (m, 2H), 7.32–7.41 (m, 3H), 7.50 (dd, J = 8.4, 0.8 Hz, 1H), 7.73 (d, J = 8.4 Hz, 1H), 7.94 (dd, J = 8.4, 1.6 Hz, 2H), 9.54 (bs, 1H); 13C NMR (100 MHz, CDCl3) δ 21.8, 112.3, 112.5, 121.1, 123.3, 126.5, 127.9, 129.3, 134.7, 135.5, 137.6, 143.3, 187.1; HRMS (ESI) calcd for C16H14NO [M + H]+ 236.1075; found 236.1071.
1); IR (KBr) 3350, 1616, 1515, 1261, 825, 750 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.48 (s, 3H), 7.14–7.20 (m, 2H), 7.32–7.41 (m, 3H), 7.50 (dd, J = 8.4, 0.8 Hz, 1H), 7.73 (d, J = 8.4 Hz, 1H), 7.94 (dd, J = 8.4, 1.6 Hz, 2H), 9.54 (bs, 1H); 13C NMR (100 MHz, CDCl3) δ 21.8, 112.3, 112.5, 121.1, 123.3, 126.5, 127.9, 129.3, 134.7, 135.5, 137.6, 143.3, 187.1; HRMS (ESI) calcd for C16H14NO [M + H]+ 236.1075; found 236.1071.
Acknowledgements
We thank DST (Project no. SB/S1/OC-72/2013) and DST nano mission (SR/NM/NS-1034/2012(G)) for financial support. G.K thanks for UGC, New Delhi for Senior Research fellowship.Notes and references
- (a) G. Dyker, Handbook of C–H Transformation: Applications in Organic Synthesis, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) J.-Q. Yu, and Z.-J. Shi, C–H activation, Springer, Berlin, Germany, 2010 Search PubMed; (c) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS PubMed.
- (a) F. Bellina and R. Rossi, Chem. Rev., 2010, 110, 1082–1146 CrossRef CAS PubMed; (b) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780–1824 CrossRef CAS PubMed; (c) L. Ackermann, Angew. Chem., Int. Ed., 2011, 50, 3842–3844 CrossRef CAS PubMed; (d) J. Wencel-Delord, T. Droege, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740–4761 RSC; (e) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215–1292 CrossRef CAS PubMed; (f) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147–1169 CrossRef CAS PubMed; (g) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074–1086 CrossRef CAS PubMed; (h) H. M. L. Davies, J. Du Bois and J.-Q. Yu, Chem. Soc. Rev., 2011, 40, 1855–1856 RSC; (i) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788–802 CrossRef CAS PubMed; (j) D. A. Colby, A. S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2012, 45, 814–825 CrossRef CAS PubMed; (k) G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651–3678 RSC; (l) D. Leow, G. Li, T.-S. Mei and J.-Q. Yu, Nature, 2012, 486, 518–522 CrossRef CAS PubMed; (m) C. Pan, X. Jia and J. Cheng, Synthesis, 2012, 677–685 CAS; (n) T.-S. Mei, L. Kou, S. Ma, K. M. Engle and J.-Q. Yu, Synthesis, 2012, 1778–1791 CAS; (o) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74–100 CrossRef CAS PubMed; (p) S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS PubMed; (q) S. Ko, B. Kang and S. Chang, Angew. Chem., Int. Ed., 2005, 44, 455–457 CrossRef CAS PubMed; (r) P. Alvarez-Bercedo, A. Flores-Gaspar, A. Correa and R. Martin, J. Am. Chem. Soc., 2010, 132, 466–467 CrossRef CAS PubMed; (s) P. Alvarez-Bercedo, A. Flores-Gaspar, A. Correa and R. Martin, Org. Lett., 2012, 14, 5234–5237 CrossRef PubMed; (t) B.-X. Tang, R.-J. Song, C.-Y. Wu, Y. Liu, M.-B. Zhou, W.-T. Wei, G.-B. Deng, D.-L. Yin and J.-H. Li, J. Am. Chem. Soc., 2010, 132, 8900–8902 CrossRef CAS PubMed.
- (a) H.-Y. Thu, W.-Y. Yu and C.-M. Che, J. Am. Chem. Soc., 2006, 128, 9048–9049 CrossRef CAS PubMed; (b) A. Garcia-Rubia, M. A. Fernandez-Ibanez, R. G. Arrayas and J. C. Carretero, Chem.–Eur. J., 2011, 17, 3567–3570 CrossRef CAS PubMed; (c) C. Wang, H. Chen, Z. Wang, J. Chen and Y. Huang, Angew. Chem., Int. Ed., 2012, 51, 7242–7245 CrossRef CAS PubMed; (d) J. Kim and S. Chang, J. Am. Chem. Soc., 2010, 132, 10272–10274 CrossRef CAS PubMed; (e) Y. Li, B.-J. Li, W.-H. Wang, W.-P. Huang, X.-S. Zhang, K. Chen and Z.-J. Shi, Angew. Chem., Int. Ed., 2011, 50, 2115–2119 CrossRef CAS PubMed; (f) X. Jia, S. Zhang, W. Wang, F. Luo and J. Cheng, Org. Lett., 2009, 11, 3120–3123 CrossRef CAS PubMed; (g) K. Padala and M. Jeganmohan, Org. Lett., 2011, 13, 6144–6147 CrossRef CAS PubMed; (h) F. W. Patureau, T. Besset and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1064–1067 CrossRef CAS PubMed.
- (a) G. Rousseau and B. Breit, Angew. Chem., Int. Ed., 2011, 50, 2450–2494 CrossRef CAS PubMed; (b) B. Liu, H.-Z. Jiang and B.-F. Shi, J. Org. Chem., 2014, 79, 1521–1526 CrossRef CAS PubMed; (c) X. Cong, J. You, G. Gao and J. Lan, Chem. Commun., 2013, 49, 662–664 RSC; (d) F. Zhang and D. R. Spring, Chem. Soc. Rev., 2014, 43, 6906–6919 RSC.
- (a) Y. Yuan, D. Chen and X. Wang, Adv. Synth. Catal., 2011, 353, 3373–3379 CrossRef CAS; (b) F. Xiao, Q. Shuai, F. Zhao, O. Basle, G. Deng and C.-J. Li, Org. Lett., 2011, 13, 1614–1617 CrossRef CAS PubMed; (c) S. Guin, S. K. Rout, A. Banerjee, S. Nandi and B. K. Patel, Org. Lett., 2012, 14, 5294–5297 CrossRef CAS PubMed; (d) Z. Yin and P. Sun, J. Org. Chem., 2012, 77, 11339–11344 CrossRef CAS PubMed; (e) Z. Xu, B. Xiang and P. Sun, RSC Adv., 2013, 3, 1679–1682 RSC; (f) H. Song, D. Chen, C. Pi, X. Cui and Y. Wu, J. Org. Chem., 2014, 79, 2955–2962 CrossRef CAS PubMed; (g) F. Xiong, C. Qian, D. Lin, W. Zeng and X. Lu, Org. Lett., 2013, 15, 5444–5447 CrossRef CAS PubMed; (h) Q. Zhang, F. Yang and Y. Wu, Chem. Commun., 2013, 49, 6837–6839 RSC; (i) W. Zhou, H. Li and L. Wang, Org. Lett., 2012, 14, 4594–4597 CrossRef CAS PubMed; (j) X. Li, X. Shi, M. Fang and X. Xu, J. Org. Chem., 2013, 78, 9499–9504 CrossRef CAS PubMed; (k) Z. Li, Z. Cui and Z.-Q. Liu, Org. Lett., 2013, 15, 406–409 CrossRef CAS PubMed.
- (a) L. Ackermann and A. V. Lygin, Org. Lett., 2011, 13, 3332–3335 CrossRef CAS PubMed; (b) C. Pan, H. Jin, P. Xu, X. Liu, Y. Cheng and C. Zhu, J. Org. Chem., 2013, 78, 9494–9498 CrossRef CAS PubMed; (c) Z. Ding and N. Yoshikai, Angew. Chem., Int. Ed., 2012, 51, 4698–4701 CrossRef CAS PubMed; (d) S. Xu, X. Huang, X. Hong and B. Xu, Org. Lett., 2012, 14, 4614–4617 CrossRef CAS PubMed.
- (a) O. Basle, J. Bidange, Q. Shuai and C.-J. Li, Adv. Synth. Catal., 2010, 352, 1145–1149 CrossRef CAS; (b) C.-W. Chan, Z. Zhou and W.-Y. Yu, Adv. Synth. Catal., 2011, 353, 2999–3006 CrossRef CAS; (c) Y. Wu, B. Li, F. Mao, X. Li and F. Y. Kwong, Org. Lett., 2011, 13, 3258–3261 CrossRef CAS PubMed; (d) C. Li, L. Wang, P. Li and W. Zhou, Chem.–Eur. J., 2011, 17, 10208–10212 CrossRef CAS PubMed; (e) F. Szabo, J. Daru, D. Simko, T. Z. Nagy, A. Stirling and Z. Novak, Adv. Synth. Catal., 2013, 355, 685–691 CrossRef CAS; (f) H. Li, P. Li and L. Wang, Org. Lett., 2013, 15, 620–623 CrossRef CAS PubMed; (g) Z. Wang, Q. Tian, X. Yu and C. Kuang, Adv. Synth. Catal., 2014, 356, 961–966 CrossRef CAS; (h) Q. Tian, P. He and C. Kuang, Org. Biomol. Chem., 2014, 12, 7474–7477 RSC; (i) X.-B. Yan, Y.-W. Shen, D.-Q. Chen, P. Gao, Y.-X. Li, X.-R. Song, X.-Y. Liu and Y.-M. Liang, Tetrahedron, 2014, 70, 7490–7495 CrossRef CAS PubMed.
- (a) L. Ackermann and A. V. Lygin, Org. Lett., 2011, 13, 3332–3335 CrossRef CAS PubMed; (b) C. Pan, H. Jin, P. Xu, X. Liu, Y. Cheng and C. Zhu, J. Org. Chem., 2013, 78, 9494–9498 CrossRef CAS PubMed; (c) Z. Ding and N. Yoshikai, Angew. Chem., Int. Ed., 2012, 51, 4698–4701 CrossRef CAS PubMed; (d) S. Xu, X. Huang, X. Hong and B. Xu, Org. Lett., 2012, 14, 4614–4617 CrossRef CAS PubMed.
- (a) C. Pan, H. Jin, X. Liu, Y. Cheng and C. Zhu, Chem. Commun., 2013, 49, 2933–2935 RSC; (b) M. Arthuis, R. Pontikis and J.-C. Florent, Org. Lett., 2009, 11, 4608–4611 CrossRef CAS PubMed; (c) Y. Goriya and C. V. Ramana, Chem. Commun., 2014, 50, 7790–7792 RSC; (d) Q.-Q. Yang, C. Xiao, L.-Q. Lu, J. An, F. Tan, B.-J. Li and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 9137–9140 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copy of all the compounds 1H and 13C spectra. See DOI: 10.1039/c4ra15162c |
| This journal is © The Royal Society of Chemistry 2015 |