Wearable silver nanowire dry electrodes for electrophysiological sensing
Amanda C. Myersab,
He Huangc and
Yong Zhu*abc
aDepartment of Mechanical and Aerospace Engineering, North Carolina State University, Raleigh, North Carolina, USA. E-mail: yong_zhu@ncsu.edu; Tel: +1 9195137735
bCenter for Advanced Self-Powered Systems of Integrated Sensors and Technologies (ASSIST), North Carolina State University, Raleigh, North Carolina, USA
cJoint Department of Biomedical Engineering, North Carolina State University, University of North Carolina at Chapel Hill, Chapel Hill, Raleigh, North Carolina, USA
First published on 14th January 2015
Abstract
We present wearable dry electrodes made of silver nanowires for electrophysiological sensing such as electrocardiography and electromyography. The dry electrodes perform as well as the Ag/AgCl wet electrodes when the subject is resting and show fewer motion artifacts, but without the electrolytic gel. The nanowire electrodes show no signs of skin irritation, which is desirable for long-term health monitoring.
Wearable sensors for health, wellness, and activity monitoring that track physiological data and sync with smart recording devices are becoming increasingly popular.1–4 In particular, long-term monitoring of electrophysiological (or bioelectronic) signals such as electrocardiograms (ECGs), electromyograms (EMGs), and electroencephalograms (EEGs) gives a wealth of physiological information that can be used to both monitor the body and diagnose and treat various ailments.5,6 However, conventional electrodes used to record these electrophysiological signals, while giving high-quality signals and suitable for short-term or clinical use, cannot be used in a long-term, wearable setting due to the addition of an electrolytic gel layer between the skin and electrode used to enhance the clarity of biopotential recordings; the gel eventually dries which irritates the skin and causes signal degradation.7,8,10
Dry electrodes are a viable alternative due to the elimination of the electrolytic gel layer. However, solid metal dry electrodes are uncomfortable to wear, have high skin-electrode impedance, and have large motion artifacts that result from the electrode slipping on the skin and from hairs between the skin and electrode.8,10
The aforementioned problems with dry electrodes can be mitigated or eliminated if the electrode has intimate, conformal contact with the skin. Recent advances in flexible and stretchable electronics3–6 have paved the way for dry electrodes. By creating a flexible/stretchable dry electrode, the issue of maintaining intimate skin contact is mitigated while also allowing for comfortable wear that does not impede the day-to-day activities of the user. A number of flexible/stretchable dry electrodes have been reported.9–22 Invasive electrodes, using microneedles,12 have good signal quality, but cannot be used long-term due to patient discomfort and high motion artifact. Among the variety of noninvasive (or surface) electrodes, some have costly fabrication methods14,15,17–19 and/or run the risk of the conductive metal (e.g., Au) delaminating from the flexible substrate, while others show large motion artifacts that can be attributed to the lack of conformal contact with skin.13,14 Conductive textile electrodes10,22 have been demonstrated, but the electrophysiological signals acquired have poor signal-to-noise ratios due to the electrodes sliding on the skin. Additionally, the biocompatibility of multiple dry surface electrodes has not been evaluated.15,20–22
In this paper, we present a silver nanowire (AgNW) based dry electrode that is noninvasive and wearable for electrophysiological sensing. The AgNWs are inlaid below the surface of an elastomeric substrate made of polydimethylsiloxane (PDMS), which prevents the NWs from delamination while creating a highly conductive surface (with constant conductivity >5000 S cm−1).23 The electrode is flexible and stretchable, which can conform to the curvilinear surfaces of the body thus reducing skin-electrode impedance and eliminating most motion artifacts. The AgNW dry electrodes perform as well as, and in some cases better than, the conventionally used Ag/AgCl wet electrodes in ECG and EMG measurements. Silver is widely used in biomedical applications due to its antibacterial properties, and reported studies on silver nanoparticles and NWs have shown that the antibacterial properties of bulk silver could translate to the nanoscale.24–26
The AgNW electrodes were fabricated following the method reported previously23,27–29 with modifications. AgNWs with average diameter of 90 nm and length of 10–60 μm, synthesized by the polyol method,30,31 were purchased from Blue Nano. After liquid PDMS was poured over the AgNW network, a metal snap that is compatible with current ECG/EMG equipment was pressed into the AgNW/PDMS mixture. After curing, the AgNW network was inlaid in PDMS and the snap is securely connected to the AgNW/PDMS network. The fabrication process is shown in Fig. 1a along with a finished electrode, Fig. 1b. Velcro straps (or tapes) were used to attach the electrodes to the wrist for ECG measurements, as shown in Fig. 1c, or to the forearm for EMG measurements.
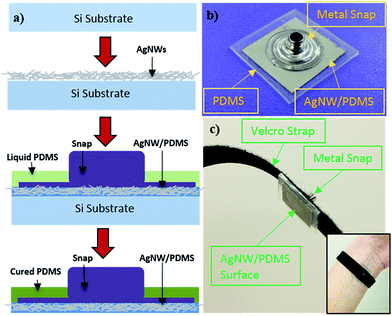 | ||
| Fig. 1 (a) Schematic of the fabrication process of the AgNW dry electrodes. (b) AgNW dry electrode with a metal snap. (c) AgNW dry electrode with Velcro strap for ECG measurements. | ||
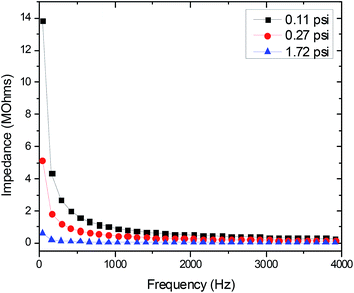
The topmost layer of skin, the stratum corneum, is considered a dielectric material with the most prominent effect on electrode-skin impedance; the drier the stratum corneum, the higher the impedance.11,32,33 While the conventional Ag/AgCl electrodes use electrolytic gel to moisten the skin and improve electrode-skin contact, dry electrodes eliminate the use of this gel. Therefore, dry electrodes need a low electrode-skin impedance to attain electrophysiological signals of comparable quality to the Ag/AgCl electrodes.11,32 The electrode-skin impedance was measured by performing a frequency sweep from 40 Hz to 100 kHz over two skin-mounted electrodes (one electrode on the left wrist and one on the right) using an impedance analyzer (4294A Precision Impedance Analyzer, Agilent). The application pressure of the electrode has a significant effect on the quality of electrode-skin contact.7,11 The electrode-skin impedance was recorded at various application pressure levels (light, medium, and heavy) to determine the proper electrode application pressure.
In addition to applying the electrolytic gel to the skin, two more steps are typically taken to treat the stratum corneum before taking an ECG with the Ag/AgCl wet electrodes to increase signal quality and improve electrode-skin contact – abrading the skin to remove dead skin cells and cleaning the electrode application area.7 However, no skin preparation was performed before the AgNW/PDMS electrodes were applied for ECG testing. Three sets of ECG data were gathered with increasing intensity of movement to study the effects of motion artifact on the electrodes. The first set of data was taken the subject was seated and resting (no movement). The second set while the subject was standing and swinging their arms (one degree of movement). And the last test was performed while the subject was jogging (two degrees of movement).
Unlike the ECG measurements, the statum corneum was only cleaned with 70% isopropyl alcohol before applying the wet or dry electrodes for the surface EMG measurement. The electrodes were placed on the right extensor digitorum communis. Two tests were performed where EMG signals were acquired: a settling trial and a wrist-extension trial. For the settling test, the subject was seated and relaxed. The right arm was placed on a flat table with a neutral/relaxed wrist position while the left arm was relaxed by the subject's side. EMG data was recorded with any muscle contractions. For the wrist-extension trial, the posture was kept the same as in the settling test. The subject performed ten wrist-extension contractions with consistent effort and approximately a 60-degree wrist extension. Each contraction lasted 3 seconds with a 10 second rest interval between each contraction. Frequency analysis was performed on the acquired EMG signals, with DC offset removed, to interpret and compare the signals gathered from each type of electrode.
Fig. 2 shows how the electrode-skin impedance changes with the application pressure. As expected, the impedance decreases with increasing pressure. This trend is attributed to the increased electrode-skin contact area with increased pressure. The medium level of pressure (0.27 psi) is most similar to the pressure applied by a wristwatch. It was also noted that increase in pressure beyond the medium level did not have a correspondingly strong effect on the reduction of skin-electrode impedance. Therefore, the medium pressure (0.27 psi) was used as the application pressure in our electrophysiological measurements.
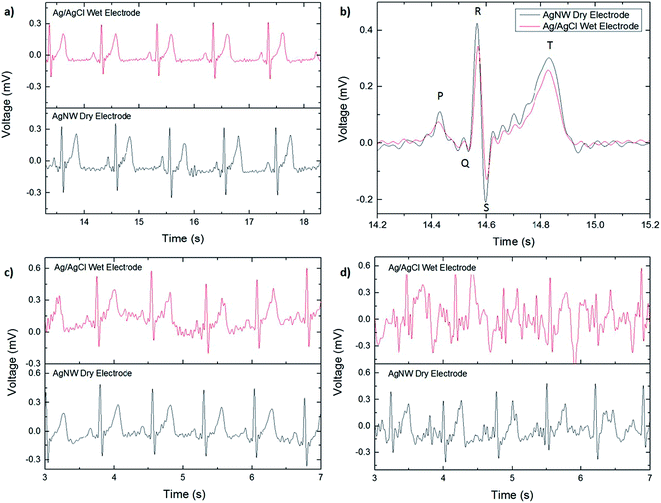
The ECGs taken with the AgNW electrode and with the Ag/AgCl electrode while the subject was resting are shown in Fig. 3a. For comparison, the ECG signals are included on the same figure although they were recorded separately. No significant differences were noted between the two, zero-degree-of-movement ECG signals. Each wave of the ECG signal (P, QRS complex, and T) are clearly defined and the absence of a wandering baseline shows that the dry electrode is well attached to the skin, as shown in Fig. 3b. No filtering in addition to the predetermined settings on the ECG amplifier was used in plotting the ECG signals, so while the signal acquired with the AgNW electrode is slightly noisier, the noise can be reduced via post-process filtering.
The effect of motion artifact on signal quality was investigated by taking ECG measurements with increasing degrees of movement. The first test, shown in Fig. 3c, consisted of localized movement near the sensing area, or one degree of movement: swinging the arms. The second test, shown in Fig. 3d, added a second degree of movement, jogging while letting the arms swing naturally. As before, the signals acquired with the AgNW electrode were measured separately from the Ag/AgCl electrode. In both tests, the AgNW electrode outperformed the Ag/AgCl electrode in terms of signal quality. This is attributed to the conformal contact of the AgNW electrode with the skin whereas the wet electrode can slide on the skin due to the gel layer. In the first test (Fig. 3c), the ECG waveform is clearly visible for both the wet and dry electrode; although the wet electrode signal has wandering baseline indicating that there is some minor slipping of the electrode on the skin. Additionally, the wet electrode shows slightly more noise in the signal than the dry electrode, making it difficult to distinguish minor nuances in the recording acquired with the wet electrode. This would pose a challenge in using the ECG signal for diagnostic purposes where clear signals are of the utmost importance. Both signals show significant degradation with the addition of a second degree of movement. For the Ag/AgCl electrode, the only discernible ECG waveform is the R peak, which could be used to determine heart rate but does not show the complete ECG spectrum. Therefore, it is unusuable in applications that require a more detailed view of the heart's performance during activity. For the AgNW electrode, the P wave, QRS complex, and T wave are still visible although the waveform has a significant amount of noise and wandering baseline.
The AgNW electrodes were able to be worn for 3 hours at a time and repeatedly for a week during a test with no noticeable discomfort or skin irritation. Degradation of AgNW electrodes over time due to oxidation could potentially compromise the device performance.34,35 Compared to unprotected AgNW electrodes (i.e., AgNWs on top), fully embedded AgNW electrodes in PDMS were found to keep the same resistance for a much longer period of time.35 In our case, the AgNW electrodes were re-used over the course of 4 months with no signal degradation.
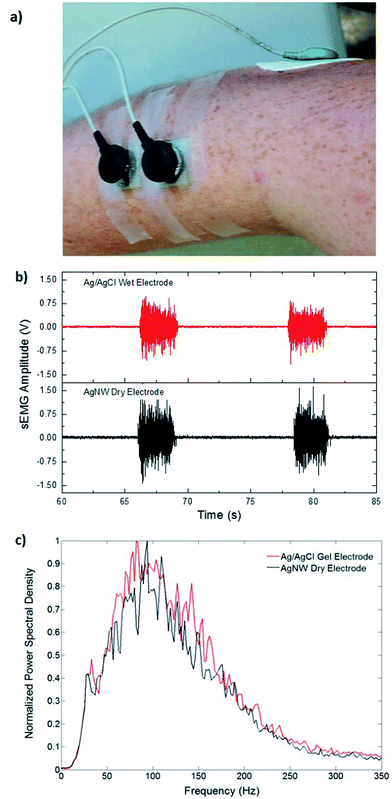
Fig. 4a shows the location of the electrodes used in the EMG measurements. Fig. 4b shows the EMG data gathered from the Ag/AgCl electrode and from the AgNW electrode during the wrist-extension trials, respectively. Visual inspection of the EMG recordings shows almost no difference between the two electrodes other than the slight amplitude increase in the AgNW recording. The EMG signal, where the subject flexed for 3 s and relaxed for 10 s, from the right extensor digitorum communis is clear in each electrode data set. While the EMG signal amplitude is higher when acquired with the AgNW electrode, the signal-to-noise ratio (SNR) of the wet Ag/AgCl electrode, 27.3 dB, is higher than that of the dry AgNW electrode, 24.7 dB. These results show that the wet and dry electrodes are comparable and that the dry AgNW electrode can be used to measure high-quality EMG recordings. The power spectral density of the each signal, shown in Fig. 4c, displays comparable spectra with the dominant frequency components of each signal residing between 25 and 180 Hz. The AgNW electrodes have mean frequency (MNF) and median frequency (MDF) values of 115.2 Hz and 135.6 Hz, respectively, while Ag/AgCl electrodes have 119.1 Hz and 139.0 Hz, respectively. The AgNW frequency values are only slightly lower than the Ag/AgCl values, again showing nearly identical electrode performance. In particular, the AgNW electrodes show promise for use in prosthetic applications as they acquire high-quality EMG signals without compromising the comfort of the wearer due to drying of the electrolytic gel or Ag/AgCl plate pressing into the skin. The AgNW electrodes can also be integrated into gel liners used in artificial limb applications.
Compared to most other dry electrodes,10,13,14,22 both the EMG and ECG signals acquired with the AgNW dry electrodes show much better quality. In addition, the AgNW electrodes generate a larger signal magnitude than the Ag/AgCl wet electrodes. Such an increase in signal magnitude can be attributed to the intimate contact between the NW electrode and the skin. The intimate contact of our AgNW electrodes is key to eliminating motion artifacts and enhancing the electrophysiological sensing capability.
In summary, wearable AgNW dry electrodes were fabricated and used to measure ECG and EMG signals with excellent performances. The electrodes are flexible and stretchable, which allows for high-quality electrophysiological measurement due to their intimate, conformal contact with the skin. When the subject was resting, ECG signals measured by the AgNW dry electrode are comparable to those by the conventional Ag/AgCl wet electrode. With increasing degrees of movement, AgNW electrodes showed less motion artifacts than the Ag/AgCl electrodes. The AgNW electrodes recorded strong, clear EMG signals with similar signal-to-noise ratio compared to the Ag/AgCl electrodes. The AgNW electrodes showed no signs of skin irritation or signal degradation after long-term wearing. In addition, the fabrication process of the AgNW electrodes is simple and cost-effective. AgNWs have been used for many stretchable and wearable device applications.36 The present study demonstrates that the AgNW dry electrodes can be an alternative to the wet electrodes in electrophysiological sensing, particularly for the long-term health monitoring.
Experimental
ECG measurements
ECG signals were measured using an ECG amplifier (ADInstruments Powerlab with ECG attachments). All ECG signals were acquired with the electrodes in the lead 1 position (negative electrode placed on the right arm, positive electrode placed on the left arm, and ground electrode placed on the right leg). Pre-gelled Ag/AgCl electrodes (Red Dot™, 3M, St. Paul, MN) were used as the commercial electrodes in ECG measurements.Surface EMG measurements
For EMG measurement, the measurement electrodes were placed in a bipolar configuration with the electrodes 22 mm apart (center-to-center), parallel to the muscle fiber direction. The ground electrode was placed on the elbow. EMG signals were sampled at 1000 Hz using a 16-channel EMG system (MA300, Motion Lab System, LA) containing a preamplifier that filtered the signals between 10 and 2000 Hz with an adjustable pass-band gain of 1000. Pre-gelled Ag/AgCl electrodes (Norotrode 20, Myotronics, Kent, WA) were used as the commercial electrodes in EMG measurements.Frequency analysis of EMG signals
For both wet and dry electrodes, the SNR is defined as
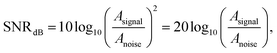 | (1) |
Both MNF and MDF were used to compare the two types of EMG electrodes.29 MNF is the sum of the product of the EMG power spectrum (P) and frequency (f) divided by the sum of the power spectrum
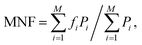 | (2) |
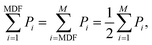 | (3) |
The power spectral density (PSD) for each muscle contraction recorded in the EMG trials was approximated from the signals without DC offset using Welch's averaged modified periodogram. The PSD values across the ten contractions were normalized between 0 and 1 according to the maximum and minimum power recorded then averaged over the 10 contractions. The MNF and MDF were then calculated using the normalized PSD values. The study protocol was approved by the Institutional Review Board at the University of North Carolina, Chapel Hill.
Acknowledgements
We gratefully acknowledge the financial support from the National Science Foundation through the ASSIST Engineering Research Center at NCSU (EEC-1160483). We thank Prof. Greg McCarty and Alison Amos for assistance with the ECG measurements, and Lin Du and Fan Zhang for assistance with the EMG measurements and analysis.Notes and references
- X.-F. Teng, Y.-T. Zhang, C. Poon and P. Bonato, IEEE Rev. Biomed. Eng., 2008, 1, 62 CrossRef PubMed.
- C. Pang, C. Lee and K.-Y. Suh, J. Appl. Polym. Sci., 2013, 130, 1429 CrossRef CAS.
- D. H. Kim, N. Lu, R. Ma, Y. S. Kim, R. H. Kim, S. Wang, J. Wu, S. M. Won, H. Tao, A. Islam, K. J. Yu, T. I. Kim, R. Chowdhury, M. Ying, L. Xu, M. Li, H. Chung, H. Keum, M. McCormick, P. Liu, Y. W. Zhang, F. G. Omenetto, Y. Huang, T. Coleman and J. A. Rogers, Science, 2011, 333, 838 CrossRef CAS PubMed.
- T. Yamada, Y. Hayamizu, Y. Yamamoto, Y. Yomogida, A. Izadi-Najafabadi, D. N. Futaba and K. Hata, Nat. Nanotechnol., 2011, 6, 296 CrossRef CAS PubMed.
- T. Sekitani, U. Zschieschang, H. Klauk and T. Someya, Nat. Mater., 2010, 9, 1015 CrossRef CAS PubMed.
- D. H. Kim, N. Lu, R. Ghaffari, Y. S. Kim, S. P. Lee, L. Xu, J. Wu, R. H. Kim, J. Song, Z. Liu, J. viventi, B. de Graff, B. Elolampi, M. Mansour, M. J. Slepian, S. Hwang, J. D. Moss, S. M. Won, Y. Huang, B. Litt and J. A. Rogers, Nat. Mater., 2011, 10, 316 CrossRef CAS PubMed.
- E. J. D. Bronzino, Medical Instrumentation: Application and Design, Wiley, Hoboken, NJ, USA, 4th edn, 2010 Search PubMed.
- A. Searle and L. Kirkup, Physiol. Meas., 2000, 21, 271 CrossRef CAS.
- Y. M. Chi, T.-P. Jung and G. Cauwenberghs, IEEE Rev. Biomed. Eng., 2010, 3, 106 CrossRef PubMed.
- P. J. Xu, H. Zhang and X. M. Tao, Text. Prog., 2008, 40, 183 CrossRef.
- A. Gruetzmann, S. Hansen and J. Müller, Physiol. Meas., 2007, 28, 1375 CrossRef PubMed.
- L. M. Yu, F. E. H. Tay, D. G. Guo, L. Xu and K. L. Yap, Sens. Actuators, A, 2009, 151, 17 CrossRef CAS PubMed.
- H.-C. Jung, J.-H. Moon, D.-H. Baek, J.-H. Lee, Y.-Y. Choi, J.-S. Hong and S.-H. Lee, IEEE Trans. Biomed. Eng., 2012, 59, 1472 CrossRef PubMed.
- J.-Y. Baek, J.-H. An, J.-M. Choi, K.-S. Park and S.-H. Lee, Sens. Actuators, A, 2008, 143, 423 CrossRef CAS PubMed.
- P. Salvo, R. Raedt, E. Carrette, D. Schaubroeck, J. Vanfleteren and L. Cardon, Sens. Actuators, A, 2012, 174, 96 CrossRef CAS PubMed.
- K. Hoffmann and R. Ruff, Proc. IEEE Eng. Med. Bio. Soc., 2007, 5739 Search PubMed.
- R. Ma, D.-H. Kim, M. McCormick, T. Coleman and J. Rogers, Proc. IEEE Eng. Med. Bio. Soc., 2010, 6405 Search PubMed.
- J.-W. Jeong, M.-K. Kim, H. Cheng, W.-H. Yeo, X. Huang, Y. Liu, Y. Zhang, Y. Huang and J. A. Rogers, Adv. Healthcare Mater., 2014, 3, 642 CrossRef CAS PubMed.
- C.-Y. Chen, C.-L. Chang, T.-F. Chien and C.-H. Luo, Sens. Actuators, A, 2013, 203, 20 CrossRef CAS PubMed.
- C.-Y. Chen, C.-L. Chang, C.-W. Chang, S.-C. Lai, T.-F. Chien, H.-Y. Huang, J.-C. Chiou and C.-H. Luo, Sensors, 2013, 13, 3077 CrossRef CAS PubMed.
- C.-T. Lin, L.-D. Liao, Y.-H. Liu, I.-J. Wang, B.-S. Lin and J.-Y. Chang, IEEE Trans. Biomed. Eng., 2011, 58, 1200 CrossRef PubMed.
- T. Oh, S. Yoon, T. Kim, H. Wi, K. Kim, E. Woo and R. Sadleir, IEEE Trans. Biomed. Cir. Sys., 2013, 7, 204 CrossRef PubMed.
- F. Xu and Y. Zhu, Adv. Mater., 2012, 24, 5117 CrossRef CAS PubMed.
- H. Johnston, G. Hutchison, F. Christensen, S. Peters, S. Hankin and V. Stone, Crit. Rev. Toxicol., 2010, 40, 328 CrossRef CAS PubMed.
- F. Christensen, H. Johnston, V. Stone, R. Aitken, S. Hankin, S. Peters and K. Aschberger, Nanotoxicology, 2010, 4, 284 CrossRef CAS PubMed.
- Y. Xiong, M. Brunson, J. Huh, A. Huang, A. Coster, K. Wendt, J. Fay and D. Qin, Small, 2013, 9, 2628 CrossRef CAS PubMed.
- S. Yao and Y. Zhu, Nanoscale, 2014, 6, 2345 RSC.
- L. Song, A. C. Myers, J. J. Adams and Y. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 4248 CAS.
- Z. Cui, F. Robles-Poblete, G. Cheng, S. Yao, X. Jiang and Y. Zhu, J. Mater. Res., 2015, 30, 79 CrossRef.
- Y. Sun, B. Mayers, T. Herricks and Y. Xia, Nano Lett., 2003, 3, 955 CrossRef CAS.
- B. Wiley, Y. Sun and Y. Xia, Acc. Chem. Res., 2007, 40, 1067 CrossRef CAS PubMed.
- S. Grimnes and Ø. G. Martinsen, Bioimpedance and Bioelectricity Basics, Academic, Boston, MA, USA, 2nd edn, 2008 Search PubMed.
- W. Franks, I. Schenker, P. Schmutz and A. Hierlemann, IEEE Trans. Biomed. Eng., 2005, 52, 1295 CrossRef PubMed.
- C.-H. Liu and X. Yu, Nanoscale Res. Lett., 2011, 6, 75 CrossRef PubMed.
- W. J. Lee, M. Y. Lee, A. K. Roy, K. S. Lee, S. Y. Park and I. In, Chem. Lett., 2013, 42, 191 CrossRef CAS.
- S. Yao and Y. Zhu, Adv. Mater., 2015 DOI:10.1002/adma.201404446 , in press.
| This journal is © The Royal Society of Chemistry 2015 |