The reaction kinetics and mechanism of crude fluoroelastomer vulcanized by direct fluorination with fluorine/nitrogen gas†
Cong Fan,
Baoyin Li,
Mengmeng Ren,
Peng Wu,
Yang Liu,
Teng Chen,
Zheng Cheng,
Jiaqiang Qin* and
Xiangyang Liu*
State Key Laboratory of Polymer Material and Engineering, College of Polymer Science and Engineering, Sichuan University, Chengdu 610065, P. R. China. E-mail: lxy6912@sina.com; jqqin@scu.edu.cn; Fax: +86 28 85405138; Tel: +86 28 85403948
First published on 28th January 2015
Abstract
A novel vulcanization method for crude fluoroelastomer by direct fluorination with fluorine/nitrogen gas has been investigated. The results show that the vulcanization reaction of fluoroelastomer is closely related with fluorination temperature, fluorination time and fluorine gas partial pressure. The maximum crosslink degree can be up to 97%, and the fluorine content of fluoroelastomer increased from 48.2% to 60% during the fluorination. The static friction coefficient of fluoroelastomer is decreased from 0.91 to 0.55, which is about 39.6% reduction after fluorination. The ATR-FTIR spectra indicate the crosslink reaction process of fluoroelastomer by direct fluorination, which arises from three reaction stages and successively goes through four elementary reactions: substitution reaction; elimination reaction; addition reaction; crosslink reaction. The increase of fluorine content takes place mainly in the first stage, and the crosslink reaction takes place mainly in the second stage and third stage.
1. Introduction
Fluoroelastomers gain increasing concern in view of their excellent thermo stability, oil resistance, mechanical property, anti-radiation performance and abrasive resistance. Most of these properties are linked to the low polarizability and the strong electronegativity of the fluorine atom, to its small van der Waals radius (0.64 Å), and to the strong C–F bond (485 kJ mol−1).7 Therefore, fluoroelastomers have extensive application in industry, including engine oil seals, fuel system components like hoses and O-rings, and drive train seals.1–6Fluoroelastomers, like all other thermosetting elastomers, need a useful and practical chemical crosslink system. The traditional vulcanization methods of fluoroelastomer mainly include ionic vulcanization and free radical vulcanization.1 During the ionic vulcanization process, HF is eliminated from –CH2–CF2– structure to form –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF– structure. Sequentially, the nucleophilic addition of a crosslinker to the –CH
CF– structure. Sequentially, the nucleophilic addition of a crosslinker to the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF– double bond proceeds, yielding crosslinks. However, the fluorine content of fluoroelastomer may decrease after ionic vulcanization. Free radical vulcanization (peroxide curing, such as a,a′-bis(t-butyl-peroxy)diisopropyl benzene and triallylisocyanurate) is a way to use free radicals to attack polymer chains to form polymeric free radicals. The polymeric radicals form crosslink networks directly or via the intermediary of radical traps. In addition, the fluorine content of fluoroelastomer still remains unchanged after free radical vulcanization.
CF– double bond proceeds, yielding crosslinks. However, the fluorine content of fluoroelastomer may decrease after ionic vulcanization. Free radical vulcanization (peroxide curing, such as a,a′-bis(t-butyl-peroxy)diisopropyl benzene and triallylisocyanurate) is a way to use free radicals to attack polymer chains to form polymeric free radicals. The polymeric radicals form crosslink networks directly or via the intermediary of radical traps. In addition, the fluorine content of fluoroelastomer still remains unchanged after free radical vulcanization.
Direct fluorination with fluorine gas has been rapidly developed in recent years.8–10 Direct fluorination reaction is usually heterogeneous, exothermic and spontaneous, owing to the high reactivity of fluorine and the formation of fluorine free radical (F˙) in the process. The F˙ can react with methylene (–CH2–) of the polymer chain to form an alkyl free radical (–CH˙–). The –CH˙– is reportedly able to form crosslink networks, according to a previous study of polyethylene fluorination.10
Therefore, it is expected that the F˙ can also be used as a new vulcanizing agent to vulcanize fluoroelastomer, and the fluorine content of fluoroelastomer increases simultaneously because the hydrogen atoms of –CH2– can be substituted by fluorine atoms during the direct fluorination. Sequentially, the properties of fluoroelastomer, such as abrasive-resistance and solvent-resistance, can be improved effectively.11 Due to the strong penetration ability of fluorine gas in rubber, not only the surface of fluoroelastomer is vulcanized but also the bulk of fluoroelastomer with sufficient thickness can be vulcanized. The abovementioned analysis inspired us to explore the possibility of fluoroelastomer vulcanized by fluorination with fluorine/nitrogen gas and the simultaneous preparation of fluoroelastomer with ultrahigh fluorine content.
In this study, the investigation on fluoroelastomer vulcanized by direct fluorination with fluorine/nitrogen gas was first reported. The fluorinated fluoroelastomer films have formed chemical crosslink networks. The fluoroelastomer shows different solubility and mechanical properties before and after fluorination. Moreover, the direct fluorination can also increase the fluorine content and decrease the static friction coefficient with no damage to the elastic property of fluoroelastomer film. The reaction kinetics and mechanism of crosslink reaction by direct fluorination have been studied systematically.
2. Experimental
2.1 Film fabrication
The crude fluoroelastomer (F26, terpolymer of vinylidene fluoride and hexafluoropropylene copolymer) was supplied by the Zhonghao Chenguang Research of Chemical Industry. 5 g crude fluoroelastomer was dissolved in 25 mL acetone, and the solution was cast on glass. Then, the films were dried at 140 °C for 6 h to remove acetone completely. The thickness of the film was controlled at about 40–50 μm.2.2 Direct fluorination
Direct fluorination was carried out in a closed stainless steel vessel. The air in the closed vessel was removed and replaced by nitrogen gas (N2 > 99.99%) for three cycles to remove residual oxygen and moisture in the chamber and on the polymer films. Then, the crude fluoroelastomer films were preheated in the closed vessel for 10 min at specific temperatures (e.g. 15 °C, 30 °C, 40 °C). After preheating, the vessel was replenished with a specific pressure of the F2/N2 (10 vol% for F2) mixed gas (e.g. 6 kPa, 10 kPa, 20 kPa) for fluorinating. The direct fluorination of fluoroelastomer films was performed at different fluorination temperatures, fluorination times and fluorine gas partial pressures to investigate the influence of these factors on the crosslink reaction of fluoroelastomer.2.3 Crosslink degree test
The fluorinated fluoroelastomer film (mass M1) was dissolved in acetone for 10 min. The insoluble part was separated from acetone, dried at 100 °C for 10 min and weighed (mass M2). The crosslink degree was calculated according to the following equation:
 | (1) |
2.4 Fluorine content tests
The bulk fluorine content of the crude and fluorinated fluoroelastomer films were tested by oxygen flask combustion (OFC) method, which is referred to in previous studies.12,13 In addition, the fluorine content of crude and fluorinated fluoroelastomer film's surface was measured by energy dispersive X-ray spectroscopy (EDX, FEI Company, USA).2.5 Characterization
The surface morphology of the crude and fluorinated fluoroelastomer film was measured by a scanning electron microscope (SEM) with FEI Inspect F (FEI company, EU/USA) at 20 kV, and the magnification was set at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×.
000×.
The tensile mechanical properties of the fluoroelastomer were measured by an Instron Model 5567 twin column table mounted testing system at ambient conditions with a gauge length of 40 mm and a crosshead speed of 50 mm min−1.
Glass transition temperature (Tg) was characterized by differential scanning calorimetry (DSC), performed on a Netzsch 204 DSC with a heating rate of 5 °C min−1 in flowing nitrogen.
The static friction coefficient of crude and fluorinated fluoroelastomer was obtained through the ratio of the maximum static friction force to the vertical gravity force.
The chemical composition and structure of fluorinated fluoroelastomer film were characterized by attenuated total reflectance Fourier transform infrared (ATR-FTIR). The ATR-FTIR spectra of fluoroelastomer films were recorded on a Nicolet Magna 650 spectroscope in the range from 4000 to 400 cm−1.
3. Results and discussion
3.1 Crosslink degree of fluorinated fluoroelastomer film
It is known that fluorination is a free radical reaction. During the polyethylene fluorination process, –CH2– structure can react with fluorine atom to develop the –CHF– structure or generate –CH˙– and F˙. The polymeric radicals of different polymer chains can be coupled to develop chemical crosslink networks.10 The crude F26 fluoroelastomer contains –CH2– structure in its macromolecular backbone that provides the possibility of a crosslink reaction. The crude fluoroelastomer can be well dissolved in acetone solvent. However, the solubility of fluoroelastomer film becomes poor in acetone after fluorination, indicating that fluoroelastomer film has developed crosslink networks during the process of direct fluorination. The crosslink degree was characterized by the solubility of fluoroelastomer in acetone after fluorination. The influences of three significant fluorination factors (fluorination temperature, fluorination time, fluorine gas partial pressure) on the crosslink degree of fluoroelastomer film were investigated.The crosslink degree of fluoroelastomer films fluorinated at different temperatures was measured, as shown in Fig. 1. The fluorine gas (F2/N2 mixed gas, 10 vol% for F2) partial pressure in the fluorination ambience was 30 kPa, and the fluorination time was 60 min. Fluoroelastomer films fluorinated at relative low fluorination temperatures (15 °C, 30 °C) have a low crosslink degree. The crosslink degree increased as the fluorination temperature increased. When the reaction took place at 30–60 °C, the crosslink degree of fluorinated films rapidly increased. The maximum value of the crosslink degree can reach 97%, when the fluoroelastomer film is fluorinated at 90 °C. At 100–140 °C fluorination, the crosslink degree declines gradually possibly due to the degradation of fluoroelastomer film. Chain degradation reaction caused by fluorine radicals rather than crosslink reaction is possibly due to the dominant reaction at 100–140 °C fluorination.
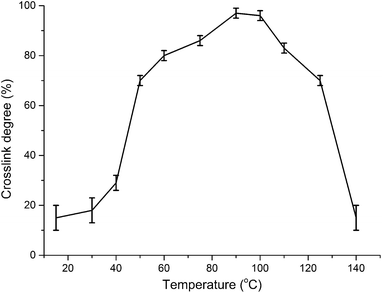 | ||
| Fig. 1 The crosslink degree as a function of fluorination temperature (°C); the fluorine gas (F2/N2 mixed gas, 10 vol% for F2) partial pressure was 30 kPa; and the fluorination time was 60 min. | ||
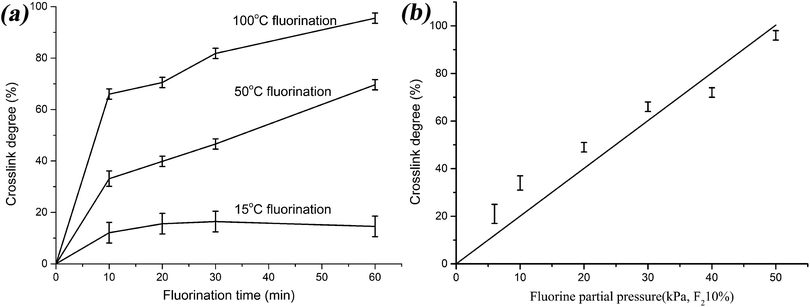
The influence of fluorination time and fluorine gas partial pressure on the crosslink degree of fluorinated films were investigated as well. As shown in Fig. 2(a), at low fluorination temperature (15 °C), the fluoroelastomer film had relatively low crosslink degree. At high fluorination temperature (50 °C, 100 °C), there is a linear relationship between crosslink degree and fluorination time after 10 min fluorination. When the fluoroelastomer film was fluorinated at 100 °C for 10 min with different fluorine gas partial pressure, there is also a linear relationship between crosslink degree and fluorine gas partial pressure, as shown in Fig. 2(b). When fluorinated with 50 kPa F2/N2 mixed gas (10 vol% for F2) for only 10 min, the fluoroelastomer film got the highest crosslink degree (96%). However, when fluorinated with 30 kPa F2/N2 mixed gas (10 vol% for F2) for a long time (60 min), the fluoroelastomer film can get the same crosslink degree (97%). The increase of F2 partial pressure can substantially shorten the fluorination time, when the fluorinated films achieve the identical crosslink degree.
It can be concluded that the fluorination temperature is the most factor that influences the crosslink reaction, which not only affects the crosslink degree but also possibly causes the chain degradation reaction. The fluorine gas partial pressure can be regarded as the second important influence factor that shortens the fluorination time.
3.2 Fluorine content analysis
The fluorine content of the fluoroelastomer was tested before and after direction fluorination by energy dispersive X-ray spectroscopy (EDX) and oxygen flask combustion (OFC) methods. The fluorine contents of crude film and fluorinated film are listed in Table 1 as weight percent (wt%). It is known that the fluorine content of rubber film's surface is tested by EDX and that of the bulk film is tested by OFC method. As shown in Table 1, after the fluorination, the fluorine contents of the film surface and the bulk film increase to 70.5 wt% and 67.7 wt% respectively, which have no significant difference between each other. Therefore, it can be concluded that the bulk of fluoroelastomer film was fluorinated rather than the surface, when it was reacted at 90 °C with 30 kPa F2/N2 mixed gas (10 vol% for F2) for 60 min. Therefore, fluorination with fluorine gas has strong penetration ability in rubber, unlike in plastic (PE, PP)10,14,15 and fiber (aramid fiber).8 The whole fluoroelastomer film (40–50 μm) can be fluorinated with fluorine gas, and the fluorinated layer in rubber is much thicker than it is in PE (0.5 μm).14 In this respect, the fluorination with fluorine gas is an effective vulcanization method for fluoroelastomer with significant increase in its fluorine content.| Method and samples | F (wt%) | |
|---|---|---|
| EDX | Crude film | 58.7 |
| Fluorinated film | 70.5 | |
| OFC | Crude film | 57.9 |
| Fluorinated film | 67.7 | |
Moreover, the fluorine content of the fluorinated film's surface at different temperature fluorination was tested by EDX, as shown in Table 2, as weight percent (wt%). The fluorine content of the fluorinated film's surface increased from 58.7 wt% to 70.7 wt% after 30 °C fluorination. Moreover, the fluorine contents kept about 70 wt% with the fluorination temperature increased from 40 °C to 90 °C. However, the crosslink degree was elevated obviously with the increase of the fluorination temperature as shown in Fig. 1. In general, the crosslink degree and the fluorine content are both increased in 30 °C fluorination. The crosslink degree increased, but the fluorine content remained unchanged in 40–90 °C fluorination. Therefore, further research is required to investigate the mechanism of the observed phenomenon.
| Untreated | 30 °C | 40 °C | 60 °C | 90 °C | |
|---|---|---|---|---|---|
| C (wt%) | 35.6 | 29.3 | 27.9 | 29.9 | 29.5 |
| F (wt%) | 58.7 | 70.7 | 72.1 | 70.1 | 70.5 |
3.3 The kinetics and mechanism of crosslink reaction by direct fluorination
As we know, direct fluorination with fluorine gas is a solid–gas reaction, which is described as a multi-stage, multi-reaction untreated shrinking core model.16–18 The untreated shrinking core model is accepted as the best simple model for majority of reacting solid–gas systems presented by Ishida in 1971.16,17 The model successfully represents the fluorination of uranium dioxide, where uranium hexafluoride gas is produced through uranyl fluoride as a solid intermediate.17 The solid–gas reaction is controlled by two main parameters: the non-dimensional diffusion rates of reactant gas and the reaction rates. When reacting for 60 min, the bulk of fluoroelastomer film was fluorinated completely, which was testified by the results of EDX and OFC methods, as shown in 3.2. Therefore, we can ignore the influence of the reactant gas diffusion in fluoroelastomer film. Only considering the reaction rates, we calculated the kinetics parameters of the fluorination with fluorine gas through the curve of crosslink degree and fluorination temperature.So the equation of fluorination reaction rate can be displayed by the Arrhenius equation:16,17
 | (2) |
 | (3) |
Thus, there is a linear relationship with ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α and 1/T. The slope indicates apparent activation energy (Ea), and the intercept represents the pre-exponential factor (A). The α, ln
α and 1/T. The slope indicates apparent activation energy (Ea), and the intercept represents the pre-exponential factor (A). The α, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α and 1/T of different fluorination temperatures are listed in Table 3 and Fig. 3.
α and 1/T of different fluorination temperatures are listed in Table 3 and Fig. 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α, 1/T of different fluorination temperatures
α, 1/T of different fluorination temperatures
| T (oC) | 15 | 30 | 40 | 50 | 60 | 75 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|
| A | 0.15 | 0.18 | 0.29 | 0.70 | 0.80 | 0.86 | 0.97 | 0.96 |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α α |
−1.90 | −1.71 | −1.24 | −0.36 | −0.22 | −0.15 | −0.04 | −0.04 |
| 1/T (×10−3 K−1) | 3.47 | 3.26 | 3.19 | 3.10 | 3.00 | 2.87 | 2.75 | 2.68 |
There are three linear relationships with ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α and 1/T in Fig. 3. Thus, the whole fluorination crosslink reaction can be divided into three stages. The apparent activation energy (Ea) and pre-exponential factor (A) of three stages are shown in Table 4.
α and 1/T in Fig. 3. Thus, the whole fluorination crosslink reaction can be divided into three stages. The apparent activation energy (Ea) and pre-exponential factor (A) of three stages are shown in Table 4.
| Reaction stage | Ea (kJ mol−1) | A (min−1) |
|---|---|---|
| Stage I | 7.4 ± 1.5 | 0.054 ± 0.003 |
| Stage II | 70.1 ± 3.4 | 2.5 ± 0.06 × 109 |
| Stage III | 6.4 ± 0.9 | 0.13 ± 0.02 |
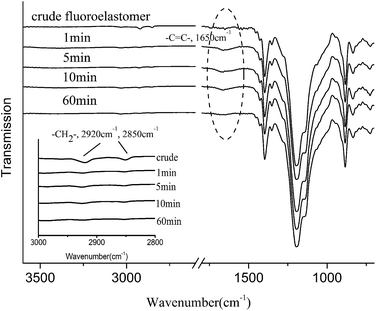
The fluorination crosslink reaction can be divided into three stages through the study of reaction kinetics. The structure changes in three reaction stages were studied by ATR-FTIR spectroscopy. Referring to Fig. 3, 30 °C fluorination was regarded as the end of stage I; 40 °C fluorination was regarded as the center of stage II; 60 °C fluorination was regarded as the start of stage III; and 90 °C fluorination was regarded as the end of stage III. Then, the ATR-FTIR spectroscopy of fluoroelastomer film fluorinated at 30 °C, 40 °C, 60 °C, and 90 °C for 60 min is shown in Fig. 4.
As shown in Fig. 4, in the crude fluoroelastomer film's ATR-FTIR spectroscopy, 2920 cm−1 and 2850 cm−1 peaks are attributed to –CH2– moieties.14,21 The –CH2– structure vanished completely in 30 °C fluorination. In addition, the fluorine content increased substantially in 30 °C fluorination as shown in Table 2, which reveals that the hydrogen atom of –CH2– structure was substituted by the fluorine atom. In the direct fluorination of polyethylene, the hydrogen atom of –CH2– is substituted by the fluorine atom at about 28 °C initially,10 and the substitution reaction of the fluorine atom occurs.
In 30–60 °C fluorination, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– (1650 cm−1)22 structure is present as shown in Fig. 4. There is no 1650 cm−1 peak in the spectroscopy of 30 °C fluorination. However, the –C
C– (1650 cm−1)22 structure is present as shown in Fig. 4. There is no 1650 cm−1 peak in the spectroscopy of 30 °C fluorination. However, the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– (1650 cm−1) structure is formed at 40 °C and 60 °C fluorination. The HF is eliminated from –CHF–CF2– structure to form –CF
C– (1650 cm−1) structure is formed at 40 °C and 60 °C fluorination. The HF is eliminated from –CHF–CF2– structure to form –CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF– structure. The 1283 cm−1, 1197 cm−1, 1137 cm−1, and 1063 cm−1 peaks are attributed to C–F moieties.10,15,18 The area of the 1063 cm−1 peak obviously decreases in 40 °C fluorination. It also indicates that there is a reaction eliminating HF.
CF– structure. The 1283 cm−1, 1197 cm−1, 1137 cm−1, and 1063 cm−1 peaks are attributed to C–F moieties.10,15,18 The area of the 1063 cm−1 peak obviously decreases in 40 °C fluorination. It also indicates that there is a reaction eliminating HF.
In 60–90 °C fluorination, the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– (1650 cm−1) structure still exists at 60 °C as shown in Fig. 7. However, at 90 °C fluorination (the crosslink degree peak at 97%), the –C
C– (1650 cm−1) structure still exists at 60 °C as shown in Fig. 7. However, at 90 °C fluorination (the crosslink degree peak at 97%), the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– (1650 cm−1) structure disappeared completely. The –C
C– (1650 cm−1) structure disappeared completely. The –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– structure reacted with fluorine (F˙) to form fluoric alkyl free radical (˙CF–CF2). Moreover, the fluoric alkyl free radical (–˙CF–CF2–) were coupled to develop a highly crosslinked network.
C– structure reacted with fluorine (F˙) to form fluoric alkyl free radical (˙CF–CF2). Moreover, the fluoric alkyl free radical (–˙CF–CF2–) were coupled to develop a highly crosslinked network.
In Fig. 4, the changes of fluoroelastomer structure in respect to the fluorination temperature were observed. To explain the mechanism of crosslink reaction by fluorination, four elementary reactions (substitution reaction, elimination reaction, addition reaction, and crosslink reaction) are presented in Scheme 1.
The short time fluorinations (fluorinated for 1 min, 5 min, 10 min at 90 °C) were processed, in order to observe the elementary reaction. The ATR-FTIR spectra of short time fluorinated films are presented in Fig. 5. At 90 °C fluorination, the –CH2– structure was substituted by fluorine atom entirely, and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– structure was generated after only one minute reaction. In view of the fact that the crosslink degree increased with the fluorination time (Fig. 2(a)), elementary reaction II (elimination reaction), elementary reaction III (addition reaction) and elementary reaction IV (crosslink reaction) are regarded as occurring concurrently at 90 °C. Moreover, one fluorine atom is eliminated in elementary reaction II and one fluorine atom would be added in elementary reaction III. Therefore, the fluorine content of fluorinated film is considered to increase after the substitution reaction (elementary reaction I) and to remain unchanged after the elimination reaction, addition reaction, and crosslink reaction.
C– structure was generated after only one minute reaction. In view of the fact that the crosslink degree increased with the fluorination time (Fig. 2(a)), elementary reaction II (elimination reaction), elementary reaction III (addition reaction) and elementary reaction IV (crosslink reaction) are regarded as occurring concurrently at 90 °C. Moreover, one fluorine atom is eliminated in elementary reaction II and one fluorine atom would be added in elementary reaction III. Therefore, the fluorine content of fluorinated film is considered to increase after the substitution reaction (elementary reaction I) and to remain unchanged after the elimination reaction, addition reaction, and crosslink reaction.
Referring to the above analysis of the elementary reactions, the fluorine content of fluorinated film is predicted to increase in stage I (only substitution reaction occurs) and remain unchanged in stage II and stage III (substitution reaction, elimination reaction, addition reaction, and crosslink reaction occur entirely). The prediction is in accordance with the fluorine content result shown in Table 2. The changes of fluorine atom content also can prove the accuracy of elementary reaction theory.
3.4 Mechanical properties
In addition, the increase of fluorine content can alter the solubility of polymers in the solvent so that fluorinated film could not be dissolved anymore. Therefore, the solubility changes of fluoroelastomer in acetone may be due to the increase of fluorine content rather than crosslinking. In this regard, the stress–strain curves of fluoroelastomer film before and after fluorination were tested. When the fluoroelastomer film was fluorinated at 90 °C with 30 kPa F2/N2 mixed gas (10 vol% for F2) for 60 min, the crosslink degree can be up to the maximum. The mechanical property of the fluorinated film prepared under the above described condition was tested. The stress–strain curves of crude and fluorinated fluoroelastomer film are shown in Fig. 6. The stress–strain curve of crude fluoroelastomer film implies that there is no crosslink network. The yield stress of crude fluoroelastomer film is only about 2.58 MPa at 120% strain. However, the stress–strain curve of fluorinated fluoroelastomer film behaves like a thermosetting elastic rubber. After the film was fluorinated at 90 °C for 60 min, the stress at 400% strain is about 4.37 MPa, indicating that there is a crosslink network in the fluorinated film.23,24 Furthermore, as shown in Fig. 1 and Table 2, when the fluorination temperature increased from 40 °C to 90 °C, the crosslink degree increases sharply and the fluorine content remained unchanged, also indicating that the solubility changes of fluoroelastomer in acetone are caused by the crosslink networks rather than the increase of fluorine content in fluorinated fluoroelastomer film.3.5 Glass-transition temperature (Tg)
In general, polymer would transform from elastomer into plastic, when its fluorine content increased substantially. The DSC curves of crude and different temperature fluorinated fluoroelastomer films are shown in Fig. 7. Moreover, Table 5 shows that the Tg of fluorinated fluoroelastomer films is slightly elevated with the increase of crosslink degree. However, the Tg of fluorinated fluoroelastomer films remains at about −23 °C, and the fluorinated films maintain the properties of the elastomer. It can be concluded that fluorination can generate the crosslink networks in fluoroelastomer, which can preserve the elastic properties simultaneously.| Samples | Tg (°C) | Crosslinking degree (%) |
|---|---|---|
| Crude film | −25.5 | 0 |
| 15 °C fluorinated film | −24.2 | 14.56 |
| 50 °C fluorinated film | −23.5 | 69.63 |
| 100 °C fluorinated film | −22.5 | 95.49 |
3.6 The friction coefficient
For elastomers, the direct fluorination with fluorine gas can decrease the friction coefficient and improve the wear life of elastomeric such as ethylene–propylene, acrylonitrile–butadiene elastomers.25 The static friction coefficient of fluorinated fluoroelastomer film prepared at different temperatures were tested and are shown in Table 6. The static friction coefficient of fluorinated film decreases from 0.91 to 0.55 at low temperature fluorination (30 °C). The static friction coefficient of crosslinking fluoroelastomer by fluorination is much lower than that of crosslinking fluoroelastomer by other methods (generally the static friction coefficient >1.0). With the increase of fluorination temperature from 30 °C to 100 °C, the static friction coefficient of fluorinated fluoroelastomer film remained at about 0.55.| Fluorination temperature | Crude film | 30 °C | 50 °C | 70 °C | 100 °C |
| Friction coefficient | 0.91 ± 0.1 | 0.55 ± 0.1 | 0.59 ± 0.1 | 0.55 ± 0.1 | 0.52 ± 0.1 |
4. Conclusions
The present study demonstrates that direct fluorination with F2/N2 mixed gas comprises a new and successful way to vulcanize fluoroelastomer. The fluoroelastomer vulcanized by direct fluorination achieves a great increase in the crosslink degree with no damage to its elastic properties when the fluorine content is significantly increased. Moreover, the static friction coefficient of fluoroelastomer film was greatly decreased. The crosslink reaction of fluoroelastomer was controlled by fluorination temperature, fluorination time and fluorine gas partial pressure. The mechanism of crosslink reaction by fluorination has been elaborated. The mechanism of crosslink reaction by fluorination mainly successively goes through four elementary reactions. First, a hydrogen atom of –CH2– is substituted by a fluorine atom. Sequentially, HF is eliminated from the polymer main chain and develops the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– structure. In the next step, F˙ and –C
C– structure. In the next step, F˙ and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– structure takes place in an additional reaction, developing the fluoric alkyl free radicals. Finally, the fluoric alkyl free radicals couple complete the crosslink. The increase of fluorine content takes place mainly in the first stage, and the crosslink reaction takes place mainly in the second stage and third stage.
C– structure takes place in an additional reaction, developing the fluoric alkyl free radicals. Finally, the fluoric alkyl free radicals couple complete the crosslink. The increase of fluorine content takes place mainly in the first stage, and the crosslink reaction takes place mainly in the second stage and third stage.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant no. 50973073) and the State Key Laboratory of Polymer Materials Engineering (Grant no. sklpme 2014-2-04). The authors acknowledge the Analytical & Testing Centre of Sichuan University, People's Republic of China, for characterization.Notes and references
- B. Ameduri, B. Boutevin and G. Kostov, Prog. Polym. Sci., 2001, 26, 105–187 CrossRef CAS.
- J. Chen, S. Tan, G. Gao, H. Li and Z. Zhang, Polym. Chem., 2014, 5, 2130–2141 RSC.
- S. Tan, J. Li, G. Gao, H. Li and Z. Zhang, J. Mater. Chem., 2012, 22, 18496–18504 RSC.
- J. Tan, Y. J. Chao, J. W. Van Zee, X. Li, X. Wang and M. Yang, Mater. Sci. Eng., A, 2008, 496, 464–470 CrossRef PubMed.
- C. Giovedi, E. Segura Pino, M. Rabello Rossi and L. Diva Brocardo Machado, Nucl. Instrum. Methods Phys. Res., Sect. B, 2007, 265, 256–259 CrossRef CAS PubMed.
- I. R. G. Ogilvie, V. J. Sieben, B. Cortese, M. C. Mowlem and H. Morgan, Lab Chip, 2011, 11, 2455–2459 RSC.
- H.-J. Schneider, Chem. Sci., 2012, 3, 1381–1394 RSC.
- J. Gao, Y. Dai, X. Wang, J. Huang, J. Yao, J. Yang and X. Liu, Appl. Surf. Sci., 2013, 270, 627–633 CrossRef CAS PubMed.
- A. P. Kharitonov, R. Taege, G. Ferrier, V. V. Teplyakov, D. A. Syrtsova and G. H. Koops, J. Fluorine Chem., 2005, 126, 251–263 CrossRef CAS PubMed.
- A. P. Kharitonov, R. Taege, G. Ferrier and N. P. Piven, Surf. Coat. Int., Part B, 2005, 88(B3), 157–230 Search PubMed.
- D. Cahard and V. Bizet, Chem. Soc. Rev., 2014, 43, 135–147 RSC.
- W. Geng, T. Nakajima, H. Takanashi and A. Ohki, Fuel, 2007, 86, 715–721 CrossRef CAS PubMed.
- W. Geng, T. Furuzono, T. Nakajima, H. Takanashi and A. Ohki, J. Hazard. Mater., 2010, 176, 356–360 CrossRef CAS PubMed.
- Z. An, C. Liu, X. Chen, F. Zheng and Y. Zhang, J. Phys. D: Appl. Phys., 2012, 45 Search PubMed.
- B. Li, C. Fan, H. Wang, M. Ren, P. Wu, X. Wang and X. Liu, RSC Adv., 2014, 4, 9321–9325 RSC.
- M. Ishida and C. Y. Wen, Chem. Eng. Sci., 1971, 26, 1031–1041 CrossRef CAS.
- S. Homma, S. Ogata, J. Koga and S. Matsumoto, Chem. Eng. Sci., 2005, 60, 4971–4980 CrossRef CAS PubMed.
- A. Amiri, G. D. Ingram, A. V. Bekker, I. Livk and N. E. Maynard, Adv. Powder Technol., 2013, 24, 728–736 CrossRef CAS PubMed.
- J. Yu, X. Zeng, J. Zhang, M. Zhong, G. Zhang, Y. Wang and G. Xu, Fuel, 2013, 103, 29–36 CrossRef CAS PubMed.
- G. Levêque and S. Abanades, Sol. Energy, 2014, 105, 225–235 CrossRef PubMed.
- Y. Wang, L. Liu, Z. Jing, J. Zhao and Y. Feng, RSC Adv., 2014, 4, 12490–12496 RSC.
- K. J. Kuhh, B. Hahn, V. Percec and M. W. Urban, Appl. Spectrosc., 1987, 41, 843–847 CrossRef.
- S. Oman and M. Nagode, Mater. Des., 2014, 60, 451–457 CrossRef PubMed.
- S. Amnuaypornsri, S. Toki, B. S. Hsiao and J. Sakdapipanich, Polymer, 2012, 53, 3325–3330 CrossRef CAS PubMed.
- V. G. Nazarov, V. P. Stolyarov, V. A. Baranov and L. A. Evlampieva, Russ. J. Gen. Chem., 2009, 79, 565–577 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra15096a |
| This journal is © The Royal Society of Chemistry 2015 |