A real-time colorimetric and ratiometric fluorescent probe for rapid detection of SO2 derivatives in living cells based on a near-infrared benzopyrylium dye†
Wenqiang Chena,
Xingjiang Liua,
Song Chenb,
Xiangzhi Song*ac and
Jian Kang*d
aCollege of Chemistry & Chemical Engineering, Central South University, 410083 Changsha, Hunan Province, P. R. China. E-mail: xzsong@csu.edu.cn; Fax: +86-731-88836954; Tel: +86-731-88836954
bCollege of Pharmacy, Qiqihar Medical University, 161006 Qiqihar, Heilongjiang Province, P. R. China
cState Key Laboratory of Fine Chemicals, Dalian University of Technology, 110624 Dalian, Liaoning Province, P. R. China
dThe Third Xiangya Hospital, Central South University, 410013 Changsha, Hunan Province, P. R. China
First published on 27th February 2015
Abstract
A benzopyrylium-based dye, Probe 1, was developed as a near-infrared fluorescent probe for the detection of SO2 derivatives (sulfite/bisulfite) in pure aqueous solution. In the presence of sulfite/bisulfite, the solution of Probe 1 displayed significant changes in its absorption (from 650 nm to 420 nm) and emission (from 690 nm to 489 nm) spectra. Probe 1 exhibits excellent selectivity and high sensitivity toward sulfite/bisulfite and the detection limit (S/N = 3) for bisulfite is as low as 10.4 nM. Specifically, Probe 1 shows a fast response time (within 10 s) toward sulfite/bisulfite, which makes it capable for real-time sensing or imaging both in vitro and in vivo assays. Probe 2, an analogue of Probe 1, as a turn-on fluorescent probe was also developed and displayed similar properties for the detection of sulfite/bisulfite. The demonstration of Probe 1 as a ratiometric fluorescent probe and Probe 2 as a turn-on fluorescent probe for the detection of SO2 derivatives was achieved in living HeLa cells and A431 cells, respectively.
Introduction
Sulphur dioxide (SO2), with a characteristic strong pungent odor, was conventionally regarded as an environmental pollutant and toxin for several decades.1 In physiological systems, SO2 mainly exists in two forms, sulfite and bisulfite (SO32−/HSO3−). Toxicological studies have implied that SO2 and its derivatives can alter the intracellular thiol levels, hinder the redox balance in cells and thereby cause severe neuron damage.2 Epidemiological studies further suggest that SO2 and its derivatives are also linked with various symptoms and diseases including many respiratory problems, lung cancer, cardiovascular diseases and neurological disorders.3 Despite that SO32−/HSO3− have toxicological effects at high concentrations, they may play important roles in many physiological processes at a physiological concentration. Generally, endogenous SO32−/HSO3− are generated from sulphur-containing amino acids such as cysteine or glutathione, which plays a crucial role in maintaining the biological sulphur balance in the body.4 Recent studies have demonstrated that SO32−/HSO3− at low concentrations (less than 450 μM) have an endothelium-dependent vasorelaxing effect and are regarded as novel messengers in cardiovascular system.5 Furthermore, some studies have found that SO2 derivatives have regulatory effects on lipid metabolism and maintain blood insulin levels.6In order to further understanding the physiological effects and detailed functions of SO32−/HSO3−, a variety of disparate approaches have been utilized for SO32−/HSO3− determination including electrochemistry, chromatography, flow injection analysis, chemiluminescence, electrochemical and enzymatic techniques.7 However, most of these conventional methods are time-consuming, have poor selectivity, require expensive instruments or involve complicated procedures, which limits their applications in biological samples. Therefore, the effective methods for rapid and accurate detection of the intracellular SO32−/HSO3− are of great importance. Owing to the operational simplicity, potential high spatial and temporal resolution, fluorescent probes have become powerful tools for visualizing morphological details and detecting biomolecules in living system.8 In the past decades, numerous fluorescent probes have been developed for detecting intracellular ions,9 small molecules10 and biomacromolecules.11 Up to now, some fluorescent probes for SO32−/HSO3− have been constructed based on the specific reactions with an aldehyde12 or levulinate group.13 However, fluorescent probes equipped with an aldehyde or levulinate group are generally subject to poor selectivity to SO32−/HSO3− over biothiols, which may be inapplicable for intracellular SO32−/HSO3− detection or imaging. Moreover, most of these reported fluorescent probes are intensity-based, which suffer from errors associated with probe concentration, environment effects and instrumental factors.14 In contrast, ratiometric fluorescent probes allow the measurement of two different emission peaks and are less susceptible to the aforementioned shortcomings.15 Therefore, new strategies are highly in demand to construct ratiometric fluorescent probes for SO2 derivatives. It was reported that SO32−/HSO3− could add to α,β-unsaturated compounds in aqueous environment due to their strong nucleophilicity.16 By employing this strategy, several ratiometric fluorescent probes have recently been developed and exhibit good sensitivity and selectivity for the detection of SO32−/HSO3−.17 However, probes that could instantaneously response to SO32−/HSO3− in pure aqueous solution are rare.18 Thus, it has remained a challenge to explore ratiometric fluorescent probes for the rapid detection of SO32−/HSO3− in pure aqueous environments.
Benzopyrylium dyes have been used as laser dyes,19 electrophotographic sensitizers20 and chemosensors21 because of their excellent photophysical properties, such as emission in near-infrared region, high absorption coefficients, relatively high fluorescence quantum yield, and sufficient stability. Recently, Guo et al. reported Probe 1 as a ratiometric fluorescent probe for the detection of H2S based on the nucleophilic addition reaction with the benzopyrylium moiety in DMF-PBS buffer solution.22 In order to obtain a good sensitivity for H2S detection, DMF is needed as a co-solvent to accelerate the addition reaction. Considering the strong nucleophilicity of SO32−/HSO3− in aqueous environment, we envisioned that Probe 1 could be a good ratiometric fluorescent probe of the detection of SO32−/HSO3− and exhibit better sensitivity toward SO32−/HSO3− than H2S in pure aqueous solution. In this work, we reported the selective and sensitive detection of SO32−/HSO3− by using Probes 1 and 2 in pure aqueous solution (shown in Scheme 1). The positive-charged benzopyrylium moiety in these two probes provides excellent water solubility and functions as the recognition group. The application of these two probes for the detection of SO32−/HSO3− in living cells was successfully demonstrated. Importantly, owning to the fast response time (within 10 s), Probe 1 was applied to a real-time monitoring of SO2 releasing in vitro assay.
Results and discussion
Optical properties of Probes 1 and 2
The absorption and emission spectra of Probes 1 and 2 (5.0 μM) in different solvents (CH3Cl, Toluene, THF, CH3CN, MeOH, DMSO) are shown in Fig. S1–S4, ESI.† Both of the absorption and emission peaks of the probes are in NIR region. In particular, the emission spectra of the probes covers most part of the biological window (650–900 nm), which is favorable for in vivo imaging. As the polarity of the solvent increases, the fluorescence efficiency of these two probes decreases. The photophysical properties of Probes 1 and 2 in pH 7.4 HEPES buffer were summarized in Table 1. Gratefully, Probes 1 and 2 exhibit good solubility in pure aqueous solution. The quantum yield of Probe 1 were determined to be 0.039. However, Probe 2 was almost non-fluorescent (Φ < 0.001). Hence, Probe 1 can be used as a ratiometric fluorescent probe and Probe 2 will be an off-on fluorescent probe. Moreover, both of Probes 1 and 2 exhibit good photostability (Fig. S5–S7, ESI†).| Compound | λabs/nma | εmax/M−1 cm−1 | λem/nmb | Φfc | Stokes shift |
|---|---|---|---|---|---|
| a The maximal absorption of the probe.b The maximal emission of the probe.c The fluorescence quantum yield of the probe by using Probe 1 (Φf = 0.34 in CH3CN) as a fluorescence standard. | |||||
| Probe 1 | 650 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
690 | 0.039 | 40 nm |
| Probe 2 | 663 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
718 | <0.001 | 55 nm |
Sensing properties of Probes 1 and 2 to SO32−/HSO3−
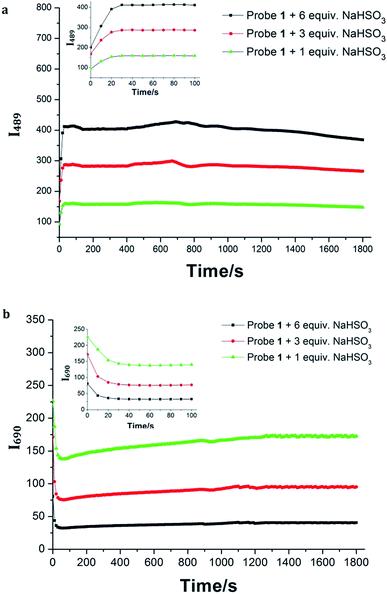
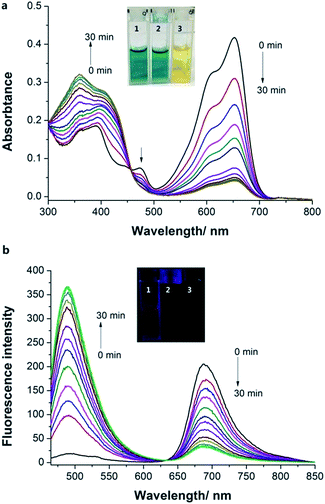
Probe 1 exhibits an absorption band with a maximum at 650 nm in HEPES buffer (20.0 mM, pH = 7.4). Upon treatment with increasing concentrations of NaHSO3 (0.0–25.0 μM), the absorption peak at 650 nm decreased gradually as well as a new peak maximizing at 421 nm concomitantly emerged (Fig. 1a). A well-defined isosbestic point at 450 nm was obtained, indicating the formation of a new compound. Meanwhile, a significant color change from blue to yellow was observed, which could be easily observed by the naked eyes. Next, the fluorescence properties of Probe 1 towards HSO3− were examined in HEPES buffer (20.0 mM, pH = 7.4). Upon excited, Probe 1 shows an emission band centered at 690 nm (Fig. 1b). In contrast, the addition of NaHSO3 to the solution of Probe 1 resulted in a new emission band centered at 489 nm. The distinct emission spectral shift (about 200 nm) is favorable for ratiometric fluorescence detection. Similar results were obtained when the solution of Probe 1 was treated with sulfite (Fig. S8, ESI†). Moreover, the emission ratio (I489/I690) of Probe 1 showed a dramatic variation (240-fold) before and after treatment with HSO3− (25.0 μM), as shown in Fig. S9, ESI.† The emission ratio of I489/I690 was proportional to the concentration of HSO3− in the range of 0.0–22.5 μM with a good linearity (R = 0.9925). The detection limit (S/N = 3) was found to be 10.4 nM for HSO3−. These results indicated that Probe 1 could detect HSO3− qualitatively and quantitatively.In addition, time-dependent fluorescence of Probe 1 in the absence/presence of HSO3− was investigated in HEPES buffer (20.0 mM, pH = 7.4). There was no obvious change in the fluorescence spectrum of Probe 1 within a reasonable measuring time, indicating that Probe 1 had good stability (Fig. S10, ESI†). However, an instant fluorescence change (less than 10 s) was observed when the solution of Probe 1 was incubated with HSO3− (20.0 μM) (Fig. 2), suggesting that Probe 1 was an ideal candidate for real-time detection of SO2 derivatives. The sensing behavior of Probe 2 toward HSO3− was also investigated under the same condition. A rapid (less than 15 s) and significant fluorescence enhancement was obtained upon the addition of NaHSO3, indicating that Probe 2 could function as an efficient turn-on fluorescent probe for HSO3−.
Mechanism studies
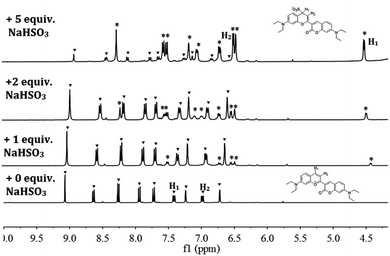
In order to provide intuitive evidence for the sensing mechanism, 1H NMR titration experiment was conducted on Probe 1 with different amounts of NaHSO3 in d6-DMSO/D2O (v/v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution (shown in Fig. 3). In the presence of NaHSO3, the signals at 7.42 ppm (H1) and 6.98 ppm (H2), assigned to the protons on the benzopyrylium moiety in Probe 1, were gradually decreased and finally disappeared while new peaks at 6.51 ppm and 4.54 ppm appeared and increased with increasing the addition amounts of NaHSO3. This result clearly indicated the formation of 1-SO3 adduct, as shown in Scheme 2. For a further confirmation, mass spectral analysis of Probe 1 with NaHSO3 in CH3CN/HEPES buffer (v/v = 1
1) solution (shown in Fig. 3). In the presence of NaHSO3, the signals at 7.42 ppm (H1) and 6.98 ppm (H2), assigned to the protons on the benzopyrylium moiety in Probe 1, were gradually decreased and finally disappeared while new peaks at 6.51 ppm and 4.54 ppm appeared and increased with increasing the addition amounts of NaHSO3. This result clearly indicated the formation of 1-SO3 adduct, as shown in Scheme 2. For a further confirmation, mass spectral analysis of Probe 1 with NaHSO3 in CH3CN/HEPES buffer (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was conducted and the appearance of the peak at m/z = 497.1727, which is almost identical to the molecular weight of 1-SO3 adduct (calcd for C26H29N2O6S = 497.1752) (Fig. S14, ESI†), provided strong support for the proposed mechanism.
1) was conducted and the appearance of the peak at m/z = 497.1727, which is almost identical to the molecular weight of 1-SO3 adduct (calcd for C26H29N2O6S = 497.1752) (Fig. S14, ESI†), provided strong support for the proposed mechanism.
Selectivity studies
To evaluate the selectivity of Probe 1 toward to SO2 derivatives, various analytes were used to investigate the fluorescence behavior of Probe 1 in HEPES buffer (20.0 mM, pH = 7.4). As shown in Fig. 4 and Fig. S15, ESI,† the addition of 25.0 μM of representative anions (F−, Cl−, Br−, I−, N3−, NO2−, NO3−, SO42−, S2O32−, AcO− and HS−), biological metal ions (Mg2+, Zn2+, Ca2+ and K+) as well as 50.0 μM of biological reactive oxygen species to the solution of Probe 1 resulted in negligible fluorescence change. It is noteworthy that the fluorescence of Probe 1 showed no changes in the presence of biological thiols (0.5 mM Cys, 0.5 mM Hcy and 4 mM GSH), which might be ascribed to the effective electrostatic repulsion between the cationic benzopyrylium moiety of Probe 1 and the cationic ammonium group of biological thiols.22 To explore the practical ability of Probe 1, we conducted competitive experiments for the detection of HSO3− with the co-existence of many potential interfering analytes. As shown in Fig. S16, ESI,† all these typically encountered analytes had little impact on the detection of HSO3−. Similarly, Probe 2 exhibited good selectivity toward SO2 derivatives (Fig. S17, ESI†).Real-time monitoring of the SO2 releasing
Fluorescent probes are indispensable tools for real-time monitoring of analytes in biology.23 Encouraged by the fast response time of Probe 1 to SO2 derivatives, we expected Probe 1 could be used for the real-time detection. Thus, a SO2 donor, sulfonamide, was prepared according to the literature method.24 SO2 can be slowly released when the SO2 donor was in the presence of Cys in pH 7.4 buffer (Fig. S18 and S19, ESI†). Thus, we treated Probe 1 (5.0 μM) with SO2 donor (50.0 μM) and Cys (500.0 μM) in HEPES buffer (containing 2% DMF) and investigated the absorption and emission spectral changes with time. As the reaction time increased, the absorption peak at 660 nm decreased and the new peak at 358 nm increased (Fig. 5a). Impressively, a time-dependent ratiometric fluorescence response was observed that the emission at 690 nm decreased and the new peak at 489 nm increased with increasing reaction time (Fig. 5b). These results demonstrated that Probe 1 could be a useful tool to monitor real-time SO2 releasing.pH studies
We also investigated pH effect on the fluorescence spectrum of Probe 1 in the absence/presence of HSO3−. The fluorescence intensity ratio (I489/I690) of Probe 1was barely affected in the pH range 2.0–11.0, which suggested that Probe 1 was stable within a wide pH range (Fig. 6). In the presence of 5.0 equiv. of HSO3−, the fluorescence intensity ratio (Mg2+, Zn2+, Ca2+ and K+) changed a little in pH range of 5.0–11.0, indicating that Probe 1 could be used for the detection of HSO3− under physiological condition.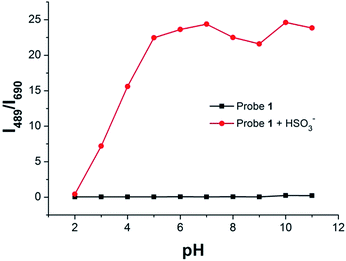 | ||
| Fig. 6 The fluorescence intensity ratio (I489/I690) of Probe 1 (5.0 μM) in the absence/presence of HSO3− (25.0 μM) at different pH values. | ||
Intracellular imaging of SO2 derivatives
Owing to the excellent sensing properties of Probes 1 and 2 for SO2 derivatives in vitro assay, we further demonstrated their capability for fluorescence imaging of SO2 derivatives in living cells (Fig. 7). HeLa cells, incubated with Probe 1 (5.0 μM) in PBS buffer (pH = 7.4) for 30 min at 37 °C, exhibited strong red fluorescence (Fig. 7c) and relatively weak green fluorescence (Fig. 7b). In contrast, when cells were pre-treated with NaHSO3 (50.0 μM) for 30 min, washed with PBS buffer (20.0 mM, pH = 7.4) and further incubated with Probe 1 (5.0 μM) for 30 min, the red fluorescence signals significantly decreased (Fig. 7f) and the green fluorescence was strongly enhanced (Fig. 7e). These fluorescence variations are in consistent with the ratiometric fluorescence responses of Probe 1 toward SO2 derivatives in vitro assay. The biological experiment indicated that Probe 1 was cell-permeable and could be used as a ratiometric fluorescent probe for real-time imaging of SO2 derivatives in living cells. In addition, Probe 2 was also used for the detection of the intracellular SO2 derivatives in living A431 cells (Fig. S22, ESI†). As expected, Probe 2 displayed the ability for the intracellular detection of SO2 derivatives in living cells. In addition, the MTT assays for Probes 1 and 2 indicated that both of the probes had minimal cytotoxicity (Fig. S23 and S24, ESI†).Experimental section
Materials and instrumentations
Unless otherwise noted, all reagents were obtained commercially and used without further purification. All solvents were dried by standard methods. Twice-distilled water was used in the experiments. NMR spectra were performed on a BRUKER 500 spectrometer, using TMS as an internal standard. Mass spectra were performed on a micrOTOF-Q II mass spectrometer (Bruker Daltonics, Germany). A Shimadzu UV-2450 spectrophotometer was used to obtain the UV-Vis absorption spectra. Fluorescence spectra were measured using a HITACHI F7000 fluorescence spectrophotometer and both of the excitation and emission slit widths were set at 5.0 nm. Unless otherwise noted, 450 nm was used as the excitation wavelength all fluorescence measurements. Cell imaging was performed on an Olympus IX83 inverted microscope. TLC silica gel plates and silica gel (mesh 200–300) for column chromatography were purchased from Qingdao Ocean Chemicals. The pH measurement was carried out on a Leici PHS-3C meter.General procedures for the spectra measurement
The stock solutions of Probe 1 were prepared at 1.0 mM in CH3CN. The solutions of various testing species were prepared from NaCl, Na2SO4, NaF, KI, NaCl, NaBr, NaN3, NaNO3, Na2S2O3·5H2O, NaNO2, CH3COONa, MgCl2, ZnCl2, CuCl2, homocysteine, GSH, cysteine, H2O2, NaClO, Na2SO3 and NaHSO3 in twice-distilled water. The test solutions of Probes 1 and 2 (5.0 μM) were prepared by placing 0.01 mL of the corresponding stock solution into 1.94 mL HEPES buffer (20.0 mM, pH = 7.4). The resulting solution was shaken well and incubated with 0.05 mL appropriate testing species for 1 min at 37 °C before recording the spectra.Cell cultures and fluorescence imaging
HeLa and A431 cells were seeded in a 12-well plate in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin. The cells were incubated under an atmosphere of 5% CO2 and 95% air at 37 °C for 24 h. Before the experiments, cells were washed with HEPES buffered solution three times. For the experiment of imaging of NaHSO3, cells were pretreated with 50.0 μM of NaHSO3 for 30 min at 37 °C for 30 min. After washing three times with HEPES buffered solution, the pretreated cells were incubated with the probes (5.0 μM) at 37 °C for additional 30 min. Fluorescence imaging was performed after washing the cells three times with HEPES buffer (20.0 mM, pH 7.4) and observed under an Olympus IX 83 fluorescence microscope.Determination of the fluorescence quantum yield
The fluorescence quantum yields of the Probes 1 and 2 were measured in HEPES buffer (20.0 mM, pH 7.4). The fluorescent quantum yield is determined using the equation:| Φ/ΦR = I/IR × ODR/OD × n2/nR2 | (1) |
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a black solid (150 mg, 57% yield). 1H NMR (500 MHz, DMSO) δ 9.07 (s, 1H), 8.64 (d, J = 8.4 Hz, 1H), 8.26 (d, J = 8.4 Hz, 1H), 7.94 (d, J = 9.4 Hz, 1H), 7.72 (d, J = 9.2 Hz, 1H), 7.42 (d, J = 9.3 Hz, 1H), 7.24 (s, 1H), 6.98 (d, J = 7.2 Hz, 1H), 6.72 (s, 1H), 3.70 (q, J = 6.9 Hz, 4H), 3.64–3.54 (m, 4H), 1.27 (t, J = 7.0 Hz, 6H), 1.19 (t, J = 7.1 Hz, 6H); 13C NMR (126 MHz, DMSO) δ 163.45, 159.16, 158.37, 156.06, 154.97, 148.68, 146.26, 133.34, 132.65, 118.09, 117.83, 112.25, 111.29, 109.96, 106.15, 96.89, 96.13, 45.96, 45.47, 12.99; ESI-HRMS: m/z calcd for C26H29N2O3: 417.2173, found: 417.2172.
1) as a black solid (150 mg, 57% yield). 1H NMR (500 MHz, DMSO) δ 9.07 (s, 1H), 8.64 (d, J = 8.4 Hz, 1H), 8.26 (d, J = 8.4 Hz, 1H), 7.94 (d, J = 9.4 Hz, 1H), 7.72 (d, J = 9.2 Hz, 1H), 7.42 (d, J = 9.3 Hz, 1H), 7.24 (s, 1H), 6.98 (d, J = 7.2 Hz, 1H), 6.72 (s, 1H), 3.70 (q, J = 6.9 Hz, 4H), 3.64–3.54 (m, 4H), 1.27 (t, J = 7.0 Hz, 6H), 1.19 (t, J = 7.1 Hz, 6H); 13C NMR (126 MHz, DMSO) δ 163.45, 159.16, 158.37, 156.06, 154.97, 148.68, 146.26, 133.34, 132.65, 118.09, 117.83, 112.25, 111.29, 109.96, 106.15, 96.89, 96.13, 45.96, 45.47, 12.99; ESI-HRMS: m/z calcd for C26H29N2O3: 417.2173, found: 417.2172.Probe 2 was prepared using the same procedure for Probe 1. Yield: 162 mg, 60%. 1H NMR (500 MHz, DMSO) δ 8.81 (s, 1H), 8.53 (d, J = 8.5 Hz, 1H), 8.17 (d, J = 8.5 Hz, 1H), 7.86 (d, J = 9.3 Hz, 1H), 7.33 (dd, J = 9.3, 2.4 Hz, 1H), 7.25 (s, 1H), 7.13 (d, J = 2.0 Hz, 1H), 3.66 (q, J = 7.0 Hz, 4H), 3.47 (dd, J = 11.8, 6.7 Hz, 4H), 2.72 (dd, J = 12.3, 6.1 Hz, 4H), 1.94–1.86 (m, 4H), 1.26 (t, J = 7.1 Hz, 6H); 13C NMR (126 MHz, DMSO) δ 163.95, 158.64, 158.50, 155.56, 153.08, 151.36, 148.09, 144.92, 132.32, 128.93, 121.88, 117.12, 117.04, 111.08, 110.22, 105.80, 103.96, 96.08, 50.88, 50.28, 45.80, 27.05, 20.70, 19.70, 19.65, 12.96; ESI-HRMS: m/z calcd for C28H29N3O3: 441.2178, found: 441.2174.
Conclusions
In conclusion, we have developed Probe 1 as a novel colorimetric and ratiometric fluorescent probe and Probe 2 as a turn-on fluorescent probe for the real-time detection of SO2 derivatives in pure aqueous environment based on a specific addition reaction. Both of these two probes displayed a fast reaction time, good sensitivity and excellent selectivity. Probe 1 displayed a significant blue shift (about 200 nm) in its fluorescence spectra in the presence of SO2 derivatives. It is noteworthy these two probes exhibited an excellent selectivity toward SO2 derivatives over other biologically relevant species. Importantly, the application of these two fluorescent probes for the detection of SO2 derivative was successfully demonstrated in living cells.Acknowledgements
The research was supported by the State Key Laboratory of Fine Chemicals (KF1202), Hunan Nonferrous Metals Holding Group Co. Ltd. and The Science and Technology projects of Hunan Province (no. 2011FJ6044 and 2010FJ4019).Notes and references
- X. Shi, J. Inorg. Biochem., 1994, 56, 155–165 CrossRef CAS.
- (a) L. Migliore and F. Coppedè, Mutat. Res., 2009, 674, 73–84 CrossRef CAS PubMed; (b) N. Sang, Y. Yun, G. Yao, H. Li, L. Guo and G. Li, Toxicol. Sci., 2011, 124, 400–413 CrossRef CAS PubMed.
- (a) S. Iwasawa, Y. Kikuchi, Y. Nishiwaki, M. Nakano, T. Michikawa, T. Tsuboi, S. Tanaka, T. Uemura, A. Ishigami and H. Nakashima, J. Occup. Health, 2009, 51, 38–47 CrossRef CAS; (b) N. Sang, Y. Yun, H. Li, L. Hou, M. Han and G. Li, Toxicol. Sci., 2010, 114, 226–236 CrossRef CAS PubMed.
- (a) M. H. Stipanuk, Annu. Rev. Nutr., 1986, 6, 179–209 CrossRef CAS PubMed; (b) M. H. Stipanuk and I. Ueki, J. Inherited Metab. Dis., 2011, 34, 17–32 CrossRef CAS PubMed.
- (a) J. Li, R. Li and Z. Meng, Eur. J. Pharmacol., 2010, 645, 143–150 CrossRef CAS PubMed; (b) X. Wang, H. Jin, C. Tang and J. Du, Eur. J. Pharmacol., 2011, 670, 1–6 CrossRef CAS PubMed.
- (a) S. S. Haider, Ind. Health, 1984, 23, 81–87 CrossRef; (b) M. Lovati, C. Manzoni, M. Daldossi, S. Spolti and C. Sirtori, Arch. Toxicol., 1996, 70, 164–173 CrossRef CAS.
- C. S. Pundir and R. Rawal, Anal. Bioanal. Chem., 2013, 405, 3049–3062 CrossRef CAS PubMed.
- (a) M. Schäferling, Angew. Chem., Int. Ed., 2012, 51, 3532–3554 CrossRef PubMed; (b) L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- (a) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2011, 112, 1910–1956 CrossRef PubMed; (b) Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2012, 113, 192–270 CrossRef PubMed.
- C. Yin, F. Huo, J. Zhang, R. Martínez-Máñez, Y. Yang, H. Lv and S. Li, Chem. Soc. Rev., 2013, 42, 6032–6059 RSC.
- (a) M. R. Gill, J. Garcia-Lara, S. J. Foster, C. Smythe, G. Battaglia and J. A. Thomas, Nat. Chem., 2009, 1, 662–667 CrossRef CAS PubMed; (b) C. Li, M. Yu, Y. Sun, Y. Wu, C. Huang and F. Li, J. Am. Chem. Soc., 2011, 133, 11231–11239 CrossRef CAS PubMed; (c) X. Peng, T. Wu, J. Fan, J. Wang, S. Zhang, F. Song and S. Sun, Angew. Chem., Int. Ed., 2011, 50, 4180–4183 CrossRef CAS PubMed.
- (a) K. Chen, Y. Guo, Z. Lu, B. Yang and Z. Shi, Chin. J. Chem., 2010, 28, 55–60 CrossRef CAS; (b) X. Cheng, H. Jia, J. Feng, J. Qin and Z. Li, Sens. Actuators, B, 2013, 184, 274–280 CrossRef CAS PubMed; (c) Y. Q. Sun, P. Wang, J. Liu, J. Zhang and W. Guo, Analyst, 2012, 137, 3430–3433 RSC; (d) G. Wang, H. Qi and X. F. Yang, Luminescence, 2013, 28, 97–101 CrossRef CAS PubMed; (e) H. Xie, F. Zeng, C. Yu and S. Wu, Polym. Chem., 2013, 4, 5416–5424 RSC; (f) X. F. Yang, M. Zhao and G. Wang, Sens. Actuators, B, 2011, 152, 8–13 CrossRef CAS PubMed; (g) Y. Yang, F. Huo, J. Zhang, Z. Xie, J. Chao, C. Yin, H. Tong, D. Liu, S. Jin and F. Cheng, Sens. Actuators, B, 2012, 166, 665–670 CrossRef PubMed; (h) C. Yu, M. Luo, F. Zeng and S. Wu, Anal. Methods, 2012, 4, 2638–2640 RSC.
- (a) S. Chen, P. Hou, J. Wang and X. Song, RSC Adv., 2012, 2, 10869–10873 RSC; (b) M. G. Choi, J. Hwang, S. Eor and S. K. Chang, Org. Lett., 2010, 12, 5624–5627 CrossRef CAS PubMed; (c) X. Gu, C. Liu, Y. Zhu and Y. Zhu, J. Agric. Food Chem., 2011, 59, 11935–11939 CrossRef CAS PubMed.
- D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596–4597 CrossRef CAS PubMed.
- (a) S. Deo and H. A. Godwin, J. Am. Chem. Soc., 2000, 122, 174–175 CrossRef CAS; (b) M. S. Tremblay, M. Halim and D. Sames, J. Am. Chem. Soc., 2007, 129, 7570–7577 CrossRef CAS PubMed.
- M. Morton and H. Landfield, J. Am. Chem. Soc., 1952, 74, 3523–3526 CrossRef CAS.
- (a) G. Li, Y. Chen, J. Wang, Q. Lin, J. Zhao, L. Ji and H. Chao, Chem. Sci., 2013, 4, 4426–4433 RSC; (b) Y. Sun, D. Zhao, S. Fan, L. Duan and R. Li, J. Agric. Food Chem., 2014, 62, 3405–3409 CrossRef CAS PubMed; (c) Y. Q. Sun, J. Liu, J. Zhang, T. Yang and W. Guo, Chem. Commun., 2013, 49, 2637–2639 RSC; (d) H. Tian, J. Qian, Q. Sun, H. Bai and W. Zhang, Anal. Chim. Acta, 2013, 788, 165–170 CrossRef CAS PubMed; (e) H. Tian, J. Qian, Q. Sun, C. Jiang, R. Zhang and W. Zhang, Analyst, 2014, 139, 3373–3377 RSC; (f) M. Y. Wu, K. Li, C. Y. Li, J. T. Hou and X. Q. Yu, Chem. Commun., 2014, 50, 183–185 RSC; (g) M. Y. Wu, T. He, K. Li, M. B. Wu, Z. Huang and X. Q. Yu, Analyst, 2013, 138, 3018–3025 RSC.
- W. Chen, Q. Fang, D. Yang, H. Zhang, X. Song and J. Foley, Anal. Chem., 2015, 87, 609–616 CrossRef CAS PubMed.
- (a) E. Brecht, Anal. Chem., 1986, 58, 384–387 CrossRef CAS; (b) P. Czerney, G. Graneß, E. Birckner, F. Vollmer and W. Rettig, J. Photochem. Photobiol., A, 1995, 89, 31–36 CrossRef CAS.
- H. Sato and M. Ikeda, J. Appl. Phys., 1972, 43, 4108–4113 CrossRef CAS PubMed.
- H. Lv, X. F. Yang, Y. Zhong, Y. Guo, Z. Li and H. Li, Anal. Chem., 2014, 86, 1800–1807 CrossRef CAS PubMed.
- J. Liu, Y. Q. Sun, J. Zhang, T. Yang, J. Cao, L. Zhang and W. Guo, Chem.–Eur. J., 2013, 19, 4717–4722 CrossRef CAS PubMed.
- (a) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC; (b) A. P. De Silva, H. N. Gunaratne, T. Gunnlaugsson, A. J. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed.
- S. R. Malwal, D. Sriram, P. Yogeeswari, V. B. Konkimalla and H. Chakrapani, J. Med. Chem., 2011, 55, 553–557 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15067h |
| This journal is © The Royal Society of Chemistry 2015 |