Stability of biodiesel, its improvement and the effect of antioxidant treated blends on engine performance and emission
Abstract
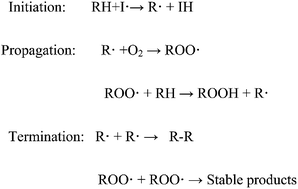
Biodiesel consists of long chain fatty acid esters derived from vegetable oils, animal fats, and used oils. Biodiesel contains different types, amounts, and configurations of unsaturated fatty acids, which are prone to oxidation. Biodiesel stability is affected by its interaction with atmospheric oxygen, light and temperature, storage conditions, and factors causing sediment formation. It can be classified broadly into three types: oxidation stability, thermal stability, and storage stability. Oxidative degradation occurs in biodiesel upon aerobic contact during storage, as well as upon contact with metal contaminants. Thermal instability focuses on the oxidation rate at higher temperatures, which is characterized by the formation of insolubles and increase in the weight of oil and fat. Storage stability is concerned with interaction between the physical and chemical characteristics of biodiesel with environmental factors, such as light, metal contamination, color changes, and sediment formation. Antioxidant concentration greatly influences engine performance and emission. The BSFC of biodiesel fuel with antioxidants is less than that of fuel without antioxidants. Moreover, an antioxidant can significantly reduce NOx formation during engine operation. Among the available synthetic antioxidants, only three antioxidants (TBHQ, PY, and PG) can significantly increase biodiesel stability. This article presents an overview of the stability of biodiesel, including the methods available for the prediction of its different stability properties. Feasible remedies to improve the stability of biodiesel and the effect of antioxidants in stabilized blends on engine performance and emission are also discussed.

 Please wait while we load your content...
Please wait while we load your content...