Synthesis of Y-shaped amphiphilic copolymers by macromolecular azo coupling reaction†
Jilei Wang,
Yuqi Zhou,
Xiaogong Wang and
Yaning He*
Department of Chemical Engineering, Key Laboratory of Advanced Materials (MOE), Tsinghua University, Beijing, 100084, P. R. China. E-mail: heyaning@mail.tsinghua.edu.cn; Fax: +86-10-62781003; Tel: +86-10-62784561
First published on 23rd December 2014
Abstract
The synthesis of AB2 Y-shaped amphiphilic block copolymers by macromolecular azo coupling reaction is reported. The hydrophobic block with the functional group at the end of the polymeric chain was prepared by controlled radical polymerization methods such as RAFT and ATRP. The hydrophilic block with diazonium salt group was obtained by the diazotization of V-shaped aniline-functionalized PEG. Then Y-shaped block copolymers were easily prepared through macromolecular azo coupling reactions between the abovementioned two polymeric blocks. The results of experiments showed that the position of the functional group suitable for azo coupling reaction in the polymeric chain has considerable effect on the efficiency of macromolecular azo coupling reaction. It was easier for the macromolecular diazonium salts to attack the functional groups when they were at the end of the macromolecular chains instead of in the middle. The self-assembly properties of the obtained Y-shaped amphiphilic block copolymers were also studied. Colloidal spheres were prepared by gradual hydrophobic aggregation of the polymeric chains in a THF–H2O dispersion medium.
Introduction
In recent years, amphiphilic block copolymers consisting of hydrophilic and hydrophobic blocks have received considerable attention for their easy self-assembly to form aggregates with different morphologies, including spherical micelles, vesicles and nanotubes, which can be potentially used in drug delivery systems, nanoreactors and others.1–10 Compared to linear amphiphilic block copolymers, Y-shaped copolymers have shown many interesting self-assembly properties due to their unique structures with three building blocks linked to a single junction point.11–14Due to their interesting properties, many efforts have been made to synthesize such Y-shaped block copolymers.15–23 For example, polymers with well-defined Y-shaped architecture can be prepared by living anionic polymerization and cationic polymerization.15–18 With the development of controlled radical polymerization (CRP), Y-shaped block copolymers can also be prepared by CRP methods such as ATRP, RAFT and NMP method.19–23 In recent years, combination of “click chemistry” and CRP methods has been proved to be an efficient platform to prepare block polymers with well-defined structures.24–29 Particularly, the 1,3-dipolar cycloaddition between one polymeric block with an azide moiety and another block with alkyne moiety catalyzed by copper(I) is mostly used to prepare block copolymers. Y-shaped block copolymers have been prepared through such click chemistry in combination with CRP methods.11,30
In addition to the copper(I) catalyzed azide–alkyne cycloaddition, Diels–Alder reactions and thiol–ene reactions have also been used as click reactions in the synthesis of block copolymers. Very recently, we have developed the macromolecular azo coupling reaction to link two polymeric blocks with high efficiency.31,32 In the past, azo coupling reaction has been widely used in the synthesis of organic azobenzene molecules or side chain azo polymers with high degree of functionalization.33–39 Our recent studies showed that well-defined diblock copolymers can be easily prepared by azo coupling reaction between macromolecular diazonium salt and polymeric block with a terminal anilino functionality in organic solvent. The introduction of terminus suitable for azo coupling reaction can be obtained by ATRP method using designed initiators or RAFT method using designed chain transfer agents.31,32
In this study, we further developed this synthetic route for the preparation of well-defined AB2 Y-shaped amphiphilic block copolymers by the combination of CRP and macromolecular azo coupling reaction. The hydrophobic polymeric block (PS or PMMA) with functional group at the end of the polymeric chain was prepared using RAFT or ATRP method. The V-shaped hydrophilic block with diazonium salt group was prepared by the diazotization of V-shaped aniline-functionalized PEG. After macromolecular azo coupling reactions between the abovementioned two polymeric blocks in DMF solvent under 0 °C, well-defined Y-shaped amphiphilic block copolymers can be prepared. The self-assembly properties of the obtained Y-shaped amphiphilic block copolymers were also studied.
Experimental
Materials and characterization
Styrene (Acros, 99%) and methyl methacrylate (Alfa Aesar, 99%) were distilled to remove the radical inhibitor before utilization. AIBN (Acros, 98%) was recrystallized twice from ethanol before utilization. All other solvents and reagents were purchased commercially and used as received.JEOL JNM-ECA 600 spectrometer was used to measure the 1HNMR and 13C NMR spectra. A gel permeation chromatography (GPC) apparatus using THF as eluent at a flow rate of 1.0 mL min−1 at 35 °C was used to measure the number average molecular weights (Mn) and molecular weight distributions (PDI), which was calibrated using linear polystyrene standards. An Agilent 8453 UV-Vis spectrophotometer was used to measure the UV-Vis spectra. Hitachi H-7650B microscope with an accelerating voltage of 80 kV was used to perform the transmission electron microscopy (TEM) observation.
Synthesis of N,N-di-(((hydroxyethoxy)ethoxy)ethyl) aniline
To a DMF (20 mL) solution containing a mixture of aniline (1.49 g, 16 mmol), K2CO3 (5.52 g, 40 mmol) and KI (1 g), 2-[2-(2-chloroethoxy)ethoxy]ethanol (6.72 g, 40 mmol) was added. The solution was stirred at 80 °C for 12 h. CH2Cl2 was added to the final solution and it was washed with H2O three times. The solution was dried with anhydrous MgSO4 and vacuumed to remove the solvent. Then column chromatography (SiO2, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate–methanol) was undertaken to obtain the final product as oil. Yield: 50%. 1H NMR (600 MHz, CDCl3) δ = 3.55–3.80 (br, 24H), 6.89 (m, 3H), 7.18 (t, 2H). 13C NMR (150 MHz, CDCl3) δ = 162.6, 147.7, 129.4, 116.2, 111.7, 72.8, 70.7, 68.7, 61.8, 50.9.
1 ethyl acetate–methanol) was undertaken to obtain the final product as oil. Yield: 50%. 1H NMR (600 MHz, CDCl3) δ = 3.55–3.80 (br, 24H), 6.89 (m, 3H), 7.18 (t, 2H). 13C NMR (150 MHz, CDCl3) δ = 162.6, 147.7, 129.4, 116.2, 111.7, 72.8, 70.7, 68.7, 61.8, 50.9.
Synthesis of ATRP initiator
To a CH2Cl2 (5 mL) solution containing a mixture of N,N-di(((hydroxyethoxy)ethoxy) ethyl) aniline (0.36 g, 1 mmol) and triethylamine (1 mL), 2-bromoisobutyryl bromide (0.92 g, 4 mmol) in 5 mL CH2Cl2 was added under ice bath. The solution was stirred at room temperature overnight. To the resulting solution CH2Cl2 was added and washed with water and dried over anhydrous MgSO4. After solvent removal, column chromatography (SiO2, ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether = 2
petroleum ether = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was undertaken to afford the product as oil. Yield: 55%. 1H NMR (600 MHz, CDCl3) δ = 1.94 (s, 12H), 3.55–3.80 (br, 20H), 4.31 (t, 4H), 6.66 (t, 1H), 6.69 (d, 2H), 7.19 (t, 2H). 13C NMR (150 MHz, CDCl3) δ = 171.7, 147.8, 129.4, 116.1, 111.8, 70.8, 68.9, 68.6, 65.2, 55.8, 51.0, 30.8.
3) was undertaken to afford the product as oil. Yield: 55%. 1H NMR (600 MHz, CDCl3) δ = 1.94 (s, 12H), 3.55–3.80 (br, 20H), 4.31 (t, 4H), 6.66 (t, 1H), 6.69 (d, 2H), 7.19 (t, 2H). 13C NMR (150 MHz, CDCl3) δ = 171.7, 147.8, 129.4, 116.1, 111.8, 70.8, 68.9, 68.6, 65.2, 55.8, 51.0, 30.8.
Synthesis of tosylate ended poly (ethylene glycol) (PEG-Ts)
Tosylate ended poly (ethylene glycol) (PEG-Ts) was synthesized in a manner similar to that reported previously.31 Poly(ethylene glycol) mono methyl ether (Mn = 1900, PDI = 1.08) was used as the starting material. PEG-Ts: Mn (NMR) = 2100, Mn (GPC) = 3700, PDI = 1.08. 1H NMR (600 MHz, DMSO-d6) δ = 2.42 (s, 3H), 3.24 (s, 3H), 3.45–3.60 (m, 180H), 4.11 (t, 2H), 7.48 (d, 2H), 7.78 (d, 2H).Synthesis of PEG-NH2
PEG-NH2 was synthesized in a modified method similar to that reported previously.31 PEG-Ts (6.3 g, 3 mmol) and p-aminobenzoic acid (1.7 g, 12 mmol) were dissolved in 50 mL DMF. Potassium carbonate (2.1 g) was added into the DMF solution. The reaction mixture was maintained at 60 °C for 24 h with stirring. Most DMF was removed by vacuum evaporation. Then, 60 mL ice water was added to the abovementioned mixture. The solution was extracted with CH2Cl2 and washed with ice water. After dried with anhydrous MgSO4 and vacuumed to remove most of the solvent, the mixture was poured into an excessive amount of cold diethyl ether. The final product was collected by filtration. Yield: 70%. Mn (NMR) = 2100, Mn (GPC) = 3700, and PDI = 1.08. 1H NMR (600 MHz, DMSO-d6) δ = 3.24 (s, 3H), 3.45–3.60 (m, 170H), 4.26 (t, 2H), 5.96 (s, 2H), 6.56 (d, 2H), 7.63 (d, 2H).Synthesis of (PEG)2-NH2
PEG-Ts (2.1 g, 1 mmol) and 5-aminoisophthalic acid (0.06 g, 0.33 mmol) were dissolved in 30 mL DMF. Potassium carbonate (0.11 g) was added into the DMF solution. The reaction mixture was kept at 60 °C for 24 h with stirring. After filtration, the solution was poured into an excessive amount of cold diethyl ether. The product was collected by filtration and recrystallization with ethanol three times. Then the final product was dried in a vacuum oven at 45 °C for 24 h. Yield: 50%. Mn (NMR) = 4000, Mn (GPC) = 7400, and PDI = 1.15. 1H NMR (600 MHz, DMSO-d6) δ = 3.24 (s, 6H), 3.45–3.80 (m, 340H), 4.37 (t, 4H), 5.77 (s, 2H), 7.41 (s, 2H), 7.66 (s, 1H).ATRP polymerization for PS with anilino functionality in the middle part of the polymer chain (PS-N(Ph)-PS)
CuBr (71.8 mg, 0.5 mmol) and 2,2-dipyridyl (156.2 mg, 1 mmol) were added to a Schlenk flask. Then, it was degassed and back-filled with argon three times. Following this step, deoxygenated ATRP initiator (82 mg, 0.13 mmol) and styrene 10 mL were added via gas-tight syringes, which had been previously purged with argon. After degassing by three freeze–pump–thaw cycles, the flask was immersed in an oil bath preheated to 110 °C. After the polymerization for 1.5 h, the reaction mixture was diluted with THF and passed through an alumina column to remove the catalyst. The filtrate was concentrated and poured into an excess amount of methanol. The precipitate was collected by filtration, washed with methanol and then dried in a vacuum oven for 24 h. Mn (GPC) = 6900, and PDI = 1.25.ATRP or RAFT polymerization for PS or PMMA with terminal anilino functionality
The synthetic detail was reported previously.31,32Typical macromolecular azo coupling reaction
PS with anilino functionality (0.02 mmol) was dissolved in 120 mL DMF at 0 °C. The diazonium salt of (PEG)2-NH2 was prepared by adding an aqueous solution of sodium nitrite (0.06 mmol in 0.2 mL of water) into a mixture of (PEG)2-NH2 (160 mg, 0.04 mmol), HCl (36%, 0.03 mL) and H2O (0.4 mL) at 0 °C. The mixture was stirred for 15 min at that temperature and then was added dropwise into the DMF solution. Then the reaction was kept at 0 °C for 72 h. Then, the solution was precipitated with plenty of water. The precipitate was collected by filtration and dried in a vacuum oven for 24 h.Self-assembly of the Y-shaped block copolymer
(PEG)2-b-PS was dissolved in anhydrous THF, which is a good solvent for all polymer blocks, at a concentration of 0.2, 0.5 and 1 mg mL−1. To prepare the self-assembly aggregates, water was gradually added to the THF solution of (PEG)2-b-PS (1 mL) under room temperature until the water content reached 50% (vol%). The polymer solutions were stirred during the water addition. After completion of the water addition, excess water was added to the suspensions to quench the structures formed. The suspensions were dialyzed against water for 72 h to remove THF before further measurements.Results and discussion
In previous study, we have tried to synthesize Y-shaped amphiphilic block copolymers by macromolecular azo coupling reaction.31 In that case, the functionalized polymer with two PS blocks (PS-N(Ph)-PS) was prepared by ATRP utilizing the functionalized initiator, which was synthesized by esterification between N,N-diethanolaniline and 2-bromoisobutyryl bromide. After the azo coupling reaction with diazonium salts of PEG-NH2, the shift in the GPC trace towards higher molecular weight was observed. Anyway, the PDI of the resulting polymer was larger than that of the precursor polymer (PS-N(Ph)-PS). The GPC results indicated that some unreacted residue (PS-N(Ph)-PS) exist after the reaction even when 10 fold PEG-N2+ was added. In this study, we tried to increase the space length between the PS part and the functional group, which is showed in Scheme 1. Based on the successful preparation of ATRP initiator, PS blocks with the functional group in the middle could be obtained by ATRP method. Anyway, after the macromolecular azo coupling reaction with diazonium salts of PEG-NH2, similar results were obtained. The GPC result showed that Y-shaped polymer had broader PDI than that of precursor PS polymer. It was slightly difficult for the macromolecular diazonium salt to attack the positions, which were in the middle of the macromolecular chain even with longer space length between the PS part and the functional group (Fig. 1). | ||
| Scheme 1 Synthetic route of Y-shaped block copolymer PEG-b-(PS)2 through macromolecular azo coupling reaction. | ||
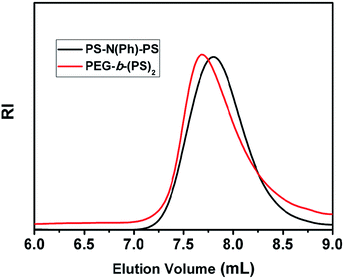 | ||
| Fig. 1 GPC traces of functionalized PS (PS-N(Ph)-PS) (PDI = 1.25, Mn = 6900) and the coupled diblock copolymer PEG-b-(PS)2 (PDI = 1.38, Mn = 7100). | ||
To prepare perfect Y-shaped amphiphilic block copolymers efficiently by macromolecular azo coupling reaction, we designed another way to prepare such Y-shaped copolymers, which is shown in Scheme 2. V-shaped aniline functionalized PEG ((PEG)2-NH2) was obtained by nucleophilic substitution reaction between synthesized PEG tosylates (PEG-Ts) and 5-aminoisophthalic acid. Because the solubility of PEG in ethanol was considerably affected by the molecular weights, the branched aniline functionalized PEG with higher molecular weight can be easily obtained by recrystallization with ethanol to remove the excess PEG-Ts. Fig. 2 shows the 1H NMR spectrum of (PEG)2-NH2. The hydrophobic block with functional group at the end of the polymeric chain was prepared by a controlled radical polymerization method such as RAFT or ATRP, which was previously reported in detail. The diazonium salt of (PEG)2-NH2 was prepared by adding NaNO2 aqueous solution into the mixture of (PEG)2-NH2 and HCl in water cooling with ice bath. Then Y-shaped block copolymers can be prepared through macromolecular azo coupling reactions between above two polymer blocks in DMF under 0 °C. In order to drive the macromolecular azo coupling reaction to completion, excessive (PEG)2-N2+ was used in the reaction. The final block copolymers were obtained by precipitation in water, and the excess of (PEG)2-N2+ can also be easily removed by plenty of water.
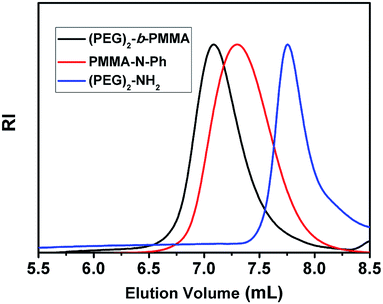
The GPC traces of (PEG)2-NH2, PMMA-N-Ph and the resulting Y-shaped diblock copolymer (PEG)2-b-PMMA are shown in Fig. 3. The calculated molecular weights of (mPEG)2-NH2, PMMA-N-Ph, and (PEG)2-b-PMMA from GPC were 7400, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 19
000, and 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively. Compared with that of the precursor polymer PMMA-N-Ph, the GPC curve of the resulting Y-shaped polymer (PEG)2-b-PMMA showed a significant shift towards higher molecular weight. The PDIs of the PMMA-N-Ph and (PEG)2-b-PMMA were 1.24 and 1.20, respectively. We also used the terminus functionalized PS to further demonstrate the applicability of this methodology. We used PS-N-Ph with different Mn prepared by different polymerization methods such as ATRP and RAFT, which have been previously reported. All the polymers showed good ability for macromolecular azo coupling reaction. After the macromolecular azo coupling reaction, the PDI of the block copolymer was similar with that of PS-N-Ph block, which are shown in Table 1.
000, respectively. Compared with that of the precursor polymer PMMA-N-Ph, the GPC curve of the resulting Y-shaped polymer (PEG)2-b-PMMA showed a significant shift towards higher molecular weight. The PDIs of the PMMA-N-Ph and (PEG)2-b-PMMA were 1.24 and 1.20, respectively. We also used the terminus functionalized PS to further demonstrate the applicability of this methodology. We used PS-N-Ph with different Mn prepared by different polymerization methods such as ATRP and RAFT, which have been previously reported. All the polymers showed good ability for macromolecular azo coupling reaction. After the macromolecular azo coupling reaction, the PDI of the block copolymer was similar with that of PS-N-Ph block, which are shown in Table 1.
The UV-Vis spectrum of the Y-shaped diblock copolymer in THF is shown in Fig. 4. The block copolymer showed evident absorption in the visible region (λmax = 426 nm), which was the typical absorption behavior of the pseudo-stilbene type of azo chromophores. The 1H NMR spectrum of (PEG)2-b-PS (Mn = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) is given in Fig. 5. The resonance at 6.0–7.0 and 3.2–3.5 were corresponding to the protons of PS part and PEG part, respectively. All the results, including GPC curves, UV-Vis spectrum and 1H NMR spectrum, indicated that all of the precursor polymers are linked with the PEG block by the azobenzene bridge to form the Y-shaped block copolymer.
000) is given in Fig. 5. The resonance at 6.0–7.0 and 3.2–3.5 were corresponding to the protons of PS part and PEG part, respectively. All the results, including GPC curves, UV-Vis spectrum and 1H NMR spectrum, indicated that all of the precursor polymers are linked with the PEG block by the azobenzene bridge to form the Y-shaped block copolymer.
The self-assembly properties of the prepared Y-shaped amphiphilic block copolymer in selected solvents were studied. Colloidal spheres were formed by gradually adding deionized water to the homogeneous solution of the Y-shaped block polymer in THF. The plot of scattered light intensity of (PEG)2-b-PS (Mn = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, PDI = 1.21) H2O–THF solutions versus the water content is shown in Fig. 6. When water was added to the (PEG)2-b-PS THF solution and reached a critical value, the PS chains would start to aggregate. The water contents at the upturning points of the curves were the critical water contents (CWCs). After this stage, as the water content further increased, the aggregation process developed gradually. Finally, when an excessive amount of water was added to the systems, the structures of self-assembled aggregates would be quenched. The CWCs were correlated with the initial concentration of the polymer, which are shown in Fig. 6. The CWCs increased with the decrease of the initial concentrations of the polymers THF solutions. TEM image of aggregates of the Y-shaped block copolymer is shown in Fig. 7. It could be found that colloidal spheres have been formed by gradual hydrophobic aggregation of the polymeric chains in a THF–H2O dispersion medium.
000, PDI = 1.21) H2O–THF solutions versus the water content is shown in Fig. 6. When water was added to the (PEG)2-b-PS THF solution and reached a critical value, the PS chains would start to aggregate. The water contents at the upturning points of the curves were the critical water contents (CWCs). After this stage, as the water content further increased, the aggregation process developed gradually. Finally, when an excessive amount of water was added to the systems, the structures of self-assembled aggregates would be quenched. The CWCs were correlated with the initial concentration of the polymer, which are shown in Fig. 6. The CWCs increased with the decrease of the initial concentrations of the polymers THF solutions. TEM image of aggregates of the Y-shaped block copolymer is shown in Fig. 7. It could be found that colloidal spheres have been formed by gradual hydrophobic aggregation of the polymeric chains in a THF–H2O dispersion medium.
 | ||
Fig. 6 Plot of scattered light intensity of (PEG)2-b-PS (Mn = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, PDI = 1.21) H2O–THF solution versus the water content, and the initial polymer concentrations in THF were 0.2, 0.5 and 1 mg mL−1. 000, PDI = 1.21) H2O–THF solution versus the water content, and the initial polymer concentrations in THF were 0.2, 0.5 and 1 mg mL−1. | ||
Conclusions
In conclusion, we have successfully developed a new approach for the synthesis of Y-shaped amphiphilic diblock copolymers by macromolecular azo coupling reaction. The hydrophobic block with functional terminus suitable for azo coupling reaction was prepared by a controlled radical polymerization method such as RAFT or ATRP. The hydrophilic block with diazonium salt group was obtained by the diazotization of V-shaped aniline-functionalized PEG. Then Y-shaped amphiphilic block copolymers with well-defined structures can be efficiently prepared through macromolecular azo coupling reactions between the abovementioned two polymer blocks in organic solvent. Colloidal spheres structures were well formed by gradually adding deionized water to the Y-shaped block polymer THF solution.Acknowledgements
This work was supported by National Natural Science Foundation of China (21474056).Notes and references
- D. M. Vriezema, M. C. Aragones, J. A. A. W. Elemans, J. J. L. M. Cornelissen, A. E. Rowan and R. J. M. Nolte, Chem. Rev., 2005, 105, 1445–1489 CrossRef CAS PubMed.
- A. Rosler, G. W. M. Vandermeulen and H. A. Klok, Adv. Drug Delivery Rev., 2012, 64, 270–279 CrossRef PubMed.
- F. H. Meng, Z. Y. Zhong and J. Feijen, Biomacromolecules, 2009, 10, 197–209 CrossRef CAS PubMed.
- H. G. Cui, Z. Y. Chen, S. Zhong, K. L. Wooley and D. J. Pochan, Science, 2007, 317, 647–650 CrossRef CAS PubMed.
- L. Zhang and A. Eisenberg, Science, 1995, 268, 1728–1731 CAS.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967–973 CrossRef CAS PubMed.
- A. Blanazs, S. P. Armes and A. J. Ryan, Macromol. Rapid Commun., 2009, 30, 267–277 CrossRef CAS PubMed.
- S. J. Holder and N. A. J. M. Sommerdijk, Polym. Chem., 2011, 2, 1018–1028 RSC.
- Z. Chen, Y. N. He, Y. Wang and X. G. Wang, Macromol. Rapid Commun., 2011, 32, 977–982 CrossRef CAS PubMed.
- N. Ma, Y. Li, H. F. Ren, H. P. Xu, Z. B. Li and X. Zhang, Polym. Chem., 2010, 1, 1609–1614 RSC.
- J. Y. Rao, Y. F. Zhang, J. Y. Zhang and S. Y. Liu, Biomacromolecules, 2008, 9, 2586–2593 CrossRef CAS PubMed.
- Y. Y. Li, X. Z. Zhang, H. Cheng, G. C. Kim, S. X. Cheng and R. X. Zhuo, Biomacromolecules, 2006, 7, 2956–2960 CrossRef CAS PubMed.
- B. Zhang, Y. P. Li, P. Ai, Z. P. Sa, Y. L. Zhao, M. Li, D. Wang and K. Sha, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5509–5526 CrossRef CAS.
- Z. B. Li, M. A. Hillmyer and T. P. Lodge, Langmuir, 2006, 22, 9409–9417 CrossRef CAS PubMed.
- N. Hadjichristidis, M. Pitsikalis, S. Pispas and H. Iatrou, Chem. Rev., 2001, 101, 3747–3792 CrossRef CAS PubMed.
- L. Yang and S. P. Gido, Macromolecules, 2001, 34, 4235–4243 CrossRef CAS.
- S. Pispas, N. Hadjichristidis, I. Potemkin and A. Khokhlov, Macromolecules, 2000, 33, 1741–1746 CrossRef CAS.
- J. Yun, R. Faust, L. S. Szilagyi, S. Keki and M. Zsuga, Macromolecules, 2003, 36, 1717–1723 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS PubMed.
- J. Liu and C. Pan, Polymer, 2005, 46, 11133–11141 CrossRef CAS PubMed.
- U. Tunca, Z. Ozyurek, T. Erdogan and G. Hizal, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 4228–4236 CrossRef CAS.
- T. He, D. Li, X. Sheng and B. Zhao, Macromolecules, 2004, 37, 3128–3135 CrossRef CAS.
- Y. Cai, Y. Tang and S. P. Armes, Macromolecules, 2004, 37, 9728–9737 CrossRef CAS.
- J.-F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018–1025 CrossRef CAS PubMed.
- C. Barner-Kowollik, F. E. Du Prez, P. Espeel, C. J. Hawker, T. Junkers, H. Schlaad and W. Van Camp, Angew. Chem., Int. Ed., 2011, 50, 60–62 CrossRef CAS PubMed.
- B. S. Sumerlin and A. P. Vogt, Macromolecules, 2010, 43, 1–13 CrossRef CAS.
- J. A. Opsteen and J. C. M. van Hest, Chem. Commun., 2005, 57–59 RSC.
- D. Quemener, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Chem. Commun., 2006, 5051–5053 RSC.
- S. Fleischmann, H. Kornber, D. Appelhans and B. I. Voit, Macromol. Chem. Phys., 2007, 208, 1050–1060 CrossRef CAS.
- L. Y. Li, W. D. He, J. A. Li, B. Y. Zhang, T. T. Pan, X. L. Sun and Z. L. Ding, Biomacromolecules, 2010, 11, 1882–1890 CrossRef CAS PubMed.
- Y. N. He, W. He, R. B. Wei, Z. Chen and X. G. Wang, Chem. Commun., 2012, 48, 1036–1038 RSC.
- Y. N. He, W. He, D. Liu, T. H. Gu, R. B. Wei and X. G. Wang, Polym. Chem., 2013, 4, 402–406 RSC.
- H. Yu and T. Ikeda, Adv. Mater., 2011, 23, 2149–2180 CrossRef CAS PubMed.
- G. Wang, X. Tong and Y. Zhao, Macromolecules, 2004, 37, 8911–8917 CrossRef CAS.
- Z. B. Li, Y. Zhang, L. R. Zhu, T. Shen and H. Q. Zhang, Polym. Chem., 2010, 1, 1501–1511 RSC.
- W. Wu, L. M. Yao, T. S. Yang, R. Y. Yin, F. Y. Li and Y. L. Yu, J. Am. Chem. Soc., 2011, 133, 15810–15813 CrossRef CAS PubMed.
- J. Barrio, L. Oriol, C. Sanchez, J. Serrano, A. Cicco, P. Keller and M. Li, J. Am. Chem. Soc., 2010, 132, 3762–3769 CrossRef PubMed.
- Y. N. He, X. G. Wang and Q. X. Zhou, Polymer, 2002, 43, 7325–7333 CrossRef CAS.
- Y. N. He, Y. Zhu, Z. Chen, W. He and X. G. Wang, Chem. Commun., 2013, 49, 5556–5558 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 13C NMR spectra, GPC traces. See DOI: 10.1039/c4ra14926b |
| This journal is © The Royal Society of Chemistry 2015 |