Chemically edge-connected multilayer graphene-based architecture with enhanced thermal stability and dispersibility: experimental evidence of making the impossible possible†
Juanjuan Gaoa,
Shupeng Zhang*a,
Xinfang Zhanga,
Chunpei Yua,
Huili Yea,
Yueyue Qiana and
Haiou Song*b
aSchool of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, China. E-mail: shupeng_2006@126.com
bState Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, China. E-mail: songhaiou2011@126.com
First published on 8th December 2014
Abstract
An edge-connected multilayer graphene-based architecture has been rationally designed for the first time by a covalent/noncovalent one-pot strategy. The novel nanohybrid exhibits enhanced thermal stability and dispersibility simultaneously, indicating the generation of increasing interfacial interactions. The successful fabrication demonstrated that the steric hindrance of each component plays an important role in synthetic reactions based on graphene oxide.
Graphene, a fascinating nanomaterial, has attracted extensive attention owing to its exceptional physicochemical properties.1–4 The rational arrangement of individual graphene sheets into specific architectures is of significance for converting the remarkable microscopic characteristics of graphene into macroscopic properties of practical application.5,6 Plenty of novel nanostructures based on graphene, such as three-dimensional (3D)-assembled graphene foams or networks, have been developed successfully, exhibiting a wonderful performance in a wide range of fields including energy conversion and storage materials,7–10 sensors,11,12 catalysis13 and composite materials,14–16 etc.17 Among them, the special building blocks with enhanced dispersibility in polymer matrix could be utilized to fabricate the corresponding nanocomposites.15 Especially, it is very low filler content that could produce a dramatic enhancement in mechanical, thermal and optical properties in comparison to the bulk polymers.1,18,19
To date, chemical functionalization of graphene by noncovalent or covalent approaches is still adopted extensively.17,20,21 The modification of graphene oxide (GO) as characteristic precursor with respect to prototype applications has been widely reported and reviewed.17,22–25 However, it is unsatisfactory that parts of the surface of GO are inaccessible to reactants because of coverage with attached substrates, implying that the limited degree of GO functionalization would become an obstacle of the full potential and efficiency of a reaction.17 Though both sides of GO are highly functionalized by oxygen-containing groups as active sites,2,26 it is still a great challenge to achieve complete delamination and functionalization by efficient chemical reactions for synthetic chemists.17 The further modification based on functionalized graphene with the assistance of introduced molecules would play a key role in performance improvements due to enhanced interfacial interaction.
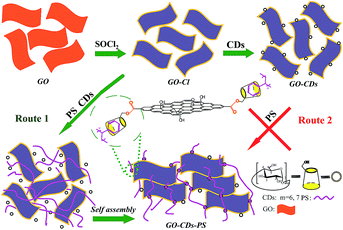
Herein, we report a rational approach for the edge-connected graphene-based frameworks by covalent/noncovalent one-pot strategy, in which the multi-layers graphene-based network is composed of multiple graphene oxide nanosheets utilizing polystyrene (PS) and cyclodextrins (CDs) as hinges. CDs are cyclic oligosaccharides composed of α(1-4)-linked D-glucopyranose units featuring a hydrophobic central cavity suitable for the stable inclusion with various organic molecules.27 In CDs, the reactive activity of primary hydroxyl groups at 6-positions is the highest.19 Furthermore, with a flexible PS as “friendly” and “soft” linkers among graphene sheets, thus the obtained PS-functionalized graphene nanocomposites could acquire better performance and suppleness as desirable for extensive applications. The fabrication process of GO–CDs–PS is illustrated in Scheme 1. To achieve the goal, two synthetic routes might be chosen. One is the conventional process (Scheme 1, Route 2), which would be step by step divided into three tortuous phases including (1) activation of GO,28 (2) CDs modified GO19 and (3) PS functionalized GO. Conversely, the other is timesaving one. After PS and CDs were simultaneously added into the activated GO solution, the final products could be obtained directly by stirring. The process was called as one-pot reaction (Scheme 1, Route 1) (the detailed experimental procedure could be found in ESI†).
More interestingly, the final products could not be achieved by the time-consuming conventional approaches from GO, GO–Cl, GO–CDs to GO–CDs–PS in the course of experiments (Route 2), indicating that ineffective or weakened molecular recognition of GO–CDs towards phenyl groups of PS. The results are attributed to the increased steric hindrance of GO–CDs, which would result in more difficult “effective collision” in organic synthesis, especially for the heterogeneous phase reaction systems based on GO. The unexpected experimental phenomenon encouraged us to explore the new method, blending the PS and CDs with GO–Cl at the same time. Fortunately, the products was obtained ultimately.
The obvious contrast for two synthetic strategy could be strongly confirmed by FT-IR spectra (Fig. 1). The FT-IR analysis of GO could be found in ESI.† As a typical example, two new bands of GO–CDβ at 1738 cm−1 and 1156 cm−1 assigned to ester carbonyl and hydroxyl groups are observed as shown in Fig. 1A(d). Besides, the IR absorptions of the CDβ at wavenumbers of 1368 cm−1 corresponding to –CH– groups is blue-shifted to 1398 cm−1 clearly. These observations could support the successful preparation of GO–CDβ.19 After functionalization by one-pot route, in the cases of GO–CDα–PS and GO–CDβ–PS, the existing peaks at 1738 cm−1, 1398 cm−1 and 1156 cm−1 indicated the unchanged structural skeleton which is the same to GO–CDs. Most importantly, two sets of new peaks at 749 cm−1, 697 cm−1 and 743 cm−1, 696 cm−1 due to –CH groups for single substituent of benzene could strongly support the presence of PS onto the GO–CDs, which are depicted in Fig. 1A(e and f) and B(d and e). However, the FT-IR spectra (Fig. 1B(a and b)) of two so-called final products prepared utilizing conventional approach instead of one pot method exhibits completely different curves. That is, the characteristic absorptions owing to –CH of PS in IR fingerprint region do not be detected (Yellow region, Fig. 1B); illustrating that PS produced hardly any modification of the GO–CDs under the conditions.
The difference of two routes could be further investigated by XRD pattern (Fig. S1†). A small changes in the position of the principal reflection for two series of CDs and PS co-functionalized GO nanomaterials were observed. The 2θ values of the products by one-pot method are always higher than that by conventional one. That means the changes of interlayer spacing might be attributed to the further functionalization of GO sheets.29 Successful PS modification could close the distance of layers, which would be the main reason of 2θ value increase. The diffuse peaks of GO–CDs–PS are located at about 25°. The new weak and broad peaks are suggesting that the samples are in a disordered state, which are very poorly ordered along the different stacking direction after treatment (Fig. S2†).30 At the same time, it should be noticed that XRD diffraction intensity of GO–CDα–PS (Fig. S2c†) is higher than that of GO–CDβ–PS (Fig. S2b†) and GO–CDα (Fig. 2), indicating that the best layer upon layer regularity of GO–CDα–PS among them is due to the enhanced interfacial interaction. Besides, as a representative example, XRD patterns of GO–CDα and GO–CDα–PS prepared by non-one pot method were also compared in Fig. 2. The positions of 2θ values are almost identical completely, demonstrating the ineffective PS modification of GO–CDα by the conventional method. It should be noticed that it is very difficult or impossible to bind the GO–CDs with PS quantitatively utilizing chemical approaches.
The enhanced interfacial interactions for PS and CDs co-modified GO could be detected via thermal gravimetric analysis (TGA) (Fig. 3). As expected, it can be seen the GO–CDα–PS have the more excellent thermal stability than that of GO–CDβ–PS, which is also attributed from more PS modified onto GO–CDs due to better host-guest recognition ability. Specifically, GO–CDβ–PS start to decompose and fall below the performance of GO–CDβ at 366 °C, an onset temperature of PS decomposition, demonstrating different degree of functionalization would directly affect the macro properties of nanocomposite materials. The introduction of CDs and PS on GO is of efficiency elevating the thermal stability of functionalized graphene due to building-up of strong inter- and intra-molecular interactions.22 Unambiguous evidence in the coexistence of PS, CDs and graphene oxide in the nano-architecture provided TGA is consistent to that of FT-IR, XRD, Raman.
Raman spectroscopy is one of the most powerful methods for characterizing graphene, GO, and their derivatives,31 which could provide information about the integrity of the carbon framework (Fig. S3 of the ESI†).32 When CDs and PS with sp3 defects are introduced into the basal plane of graphene oxide, all the bands broaden and the ID/IG ratio increases from 1.01 to about 1.24–1.32. It is notable that ID/IG ratio of GO–CDβ–PS is less than that of GO–CDβ due to introduction of benzene groups with delocalized conjugated structure. And for exactly that reason, ID/IG ratio of GO–CDβ–PS is higher than that of GO–CDα–PS, suggesting the better degree of functionalization32 owing to more PS loading onto GO–CDα.
Scanning electron microscopy (SEM) of GO and its derivatives are showed in Fig. 4 and S4.† The formation of the interesting structures in the composite as inferred from the XRD data above should be reflected in the SEM images. The morphology of GO in Fig. S4(a and b†) is observed to be a face-to-face stacking of flaky texture, illustrating its layered microstructure. Comparing with GO, the stacking of every layers of GO–CDβ as a typical example are much desultory and irregular (Fig. 4a and S4c†).19 As for the final products, the edge-connected multilayer graphene-based architecture has been built by piling up individual CDs functionalized graphene (GO–CDs) with the assistance of PS based on supermolecular assembly (Fig. 4b and c, 1 and 3). Especially, the higher regularity and thickness of GO–CDα–PS strongly suggested the better degree of functionalization than that of GO–CDβ–PS. These phenomena are consistent with the results of TGA and XRD demonstrated mentioned above.
The successful reaction procedures well-known from organic chemistry can be often evaluated, for example, by the dispersibility or the performance of the materials in applications. Many types of reactions were also applied to introduce functional molecules on graphene so as to generate new properties including the formation of dispersions.33,34 In order to investigate the influence of edge-connected multilayer graphene-based in dispersibility and compatibility, approximately 1 mg of power was added to a given volume of solvent (∼1 mL), in such a way that the resulting nominal concentration was adjusted to 1 mg mL−1 for all cases. Noteworthy that our PS/CDs modified GO derivates (GO–CDα–PS and GO–CDβ–PS) can be not only dispersible in water, but also in some organic solvents, even in the nonpolar and nonprotonic ones (Fig. 5a and b). The closer structure of GO–CDα–PS resulted in the difference of dispersibility in n-hexane with GO–CDβ–PS. However, GO–CDβ could not almost be dispersed in n-hexane and toluene (Fig. 5d). Compared to GO, GO–CDs–PS has better water solubility and can be applied in more systems. The compatibility of the nanofillers impact strongly the physical/chemical properties of polymer nanocomposites.1 The high quality and uniform thin films could easily obtained by filling them in the PS matrix (Fig. 5e and f). The visualized performance could directly support the correctness of evidences of FT-IR, XRD, SEM etc. mentioned above.
In organic synthesis, the size and steric hindrance of substituent of organic molecules play a crucial factor to affect the chemical activity between two molecules, which would directly result in success or failure of the reaction. That is because the higher energy with slower reaction rate would be required due to the approach of larger atoms or groups in a chemical reaction, in comparison to a similar reaction involving smaller atoms or groups.
Almost all the chemical reactions based on GO belong to heterogeneous phase reaction systems owing to rigid structure of graphene. In the article, the rigid graphene-based nanostructure would be utilized for two times (GO–Cl and GO–CDs) during the conventional method (Scheme 1). In addition, CDs located at the edge of GO–CDs, the active sites in the next reaction, might be wrapped into the layers owing to establishment of more hydrogen bonds. Conversely, only GO–Cl would be used in the one-pot method. So, the preparation process by one-pot method could be successfully performed under the reaction conditions. Certainly, we do not deny that the successful possibility of the reaction by conventional route with the unrestricted increase of reaction temperature.
A nonsupported single graphene cannot be expected to exist, since at least a partial restacking to graphite will take place! A possibility of stabilizing individualized graphene is to “mask” the surface through chemical functionalization.35 The knowledge generated by the systematic functionalization of graphene could be a very valuable basis for exploring the chemistry of other sheet materials such as MoS2 or even so far unknown synthetic carbon allotropes.17 Therefore, as an example demonstrated in this study, these graphene frameworks as building blocks for construction of large-scale polymer nanocomposites provide a unique platform for design and development of novel graphene materials for various new practical applications.
Acknowledgements
This work was supported by the Natural Science Foundation of China (51402151, 51408297) and the Zijin Intelligent Program, Nanjing University of Science and Technology. The Natural Science Foundation of Jiangsu province (BK20130575) and Major Science and Technology Program for Water Pollution Control and Treatment, PR China (no. 2014ZX07214-001), NUST Graduate Innovation Project (no. 149).Notes and references
- H. Kim, A. A. Abdala and C. W. Macosko, Macromolecules, 2010, 43, 6515–6530 CrossRef CAS.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- V. Singh, D. Joung, L. Zhai, S. Das, S. I. Khondaker and S. Seal, Prog. Mater. Sci., 2011, 56, 1178–1271 CrossRef CAS PubMed.
- M. H. Rümmeli, C. G. Rocha, F. Ortmann, I. Ibrahim, H. Sevincli, F. Börrnert, J. Kunstmann, A. Bachmatiuk, M. Pötsche, M. Shiraishi, M. Meyyappan, B. Büchner, S. Roche and G. Cuniberti, Adv. Mater., 2011, 23, 4471 CrossRef.
- Y. Zhao, C. Hu, L. Song, L. Wang, G. Shi, L. Dai and L. Qu, Energy Environ. Sci., 2014, 7, 1913 CAS.
- Y. Zhao, C. Hu, Y. Hu, H. Cheng, G. Shi and L. Qu, Angew. Chem., Int. Ed., 2012, 51, 11371–11375 CrossRef CAS PubMed.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- B. Xu, S. Yue, Z. Sui, X. Zhang, S. Hou, G. Cao and Y. Yang, Energy Environ. Sci., 2011, 4, 2826 CAS.
- X. Wan, Y. Huang and Y. Chen, Acc. Chem. Res., 2012, 45, 598–607 CrossRef CAS PubMed.
- Y. Q. Sun, Q. O. Wu and G. Q. Shi, Energy Environ. Sci., 2011, 4, 1113–1132 CAS.
- Y. Liu, X. Dong and P. Chen, Chem. Soc. Rev., 2012, 41, 2283 RSC.
- Q. He, S. Wu, Z. Yin and H. Zhang, Chem. Sci., 2012, 3, 1764 RSC.
- C. Huang, C. Li and G. Shi, Energy Environ. Sci., 2012, 5, 8848 CAS.
- X. Sun, H. Sun, H. Li and H. Peng, Adv. Mater., 2013, 25, 153 CrossRef.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666 RSC.
- K. W. Putz, O. C. Compton, M. J. Palmeri, S. T. Nguyen and L. C. Brinson, Adv. Funct. Mater., 2010, 20, 3322–3329 CrossRef CAS.
- S. Eigler and A. Hirsch, Angew. Chem., Int. Ed., 2014, 53, 7720 CrossRef CAS PubMed.
- T. Kuilla, S. Bhadra, D. Yao, N. H. Kim, S. Bose and J. H. Lee, Prog. Polym. Sci., 2010, 35, 1350 CrossRef CAS PubMed.
- S. P. Zhang, B. Liu, C. Y. Li, W. Chen, Z. J. Yao, D. T. Yao, R. B. Yu and H.-O. Song, Chin. Chem. Lett., 2014, 25, 355 CrossRef CAS PubMed.
- Y. T. Liu, J. M. Yang, X. M. Xie and X. Y. Ye, Mater. Chem. Phys., 2011, 130, 794–799 CrossRef CAS PubMed.
- M. Fang, K. Wang, H. Lu, Y. Yang and S. Nutt, J. Mater. Chem., 2009, 19, 7098 RSC.
- S. P. Zhang, P. Xiong, X. J. Yang and X. Wang, Nanoscale, 2011, 3, 2169 RSC.
- L. Yan, Y. B. Zheng, F. Zhao, S. Li, X. Gao, B. Xu, P. S. Weiss and Y. Zhao, Chem. Soc. Rev., 2012, 41, 97 RSC.
- S. P. Zhang and H. O. Song, New J. Chem., 2012, 36(9), 1733 RSC.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906 CrossRef CAS PubMed.
- A. Lerf, H. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477 CrossRef CAS.
- Y. Tao, J. Dai, Y. Kong and Y. Sha, Anal. Chem., 2014, 86, 2633 CrossRef CAS PubMed.
- S. Niyogi, E. Bekyarova, M. E. Itkis, J. L. McWilliams, M. A. Hamon and R. C. Haddon, J. Am. Chem. Soc., 2006, 128, 7720–7721 CrossRef CAS PubMed.
- J. Shen, N. Li, M. Shi, Y. Hu and M. Ye, J. Colloid Interface Sci., 2010, 348, 377–383 CrossRef CAS PubMed.
- C. Nethravathi and M. Rajamathi, Carbon, 2008, 46, 1994–1998 CrossRef CAS PubMed.
- A. C. Ferrari and D. M. Basko, Nat. Nanotechnol., 2013, 8, 235–246 CrossRef CAS PubMed.
- J. M. Englert, P. Vecera, K. C. Knirsch, R. A. Schäfer, F. Hauke and A. Hirsch, ACS Nano, 2013, 7, 5472 CrossRef CAS PubMed.
- X. Zhong, J. Jin, S. Li, Z. Niu, W. Hu, R. Li and J. Ma, Chem. Commun., 2010, 46(39), 7340 RSC.
- L. H. Liu, M. M. Lerner and M. Yan, Nano Lett., 2010, 10, 3754–3756 CrossRef CAS PubMed.
- J. M. Englert, J. Röhrl, C. D. Schmidt, R. Graupner, M. Hundhausen, F. Hauke and A. Hirsch, Adv. Mater., 2009, 21, 4265 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, XRD patterns, Raman spectra and SEM images. See DOI: 10.1039/c4ra14891f |
| This journal is © The Royal Society of Chemistry 2015 |