BODIPY-based fluorescent probe for the simultaneous detection of glutathione and cysteine/homocysteine at different excitation wavelengths†
Li-Ya Niua,
Qing-Qing Yangac,
Hai-Rong Zhengac,
Yu-Zhe Chena,
Li-Zhu Wua,
Chen-Ho Tunga and
Qing-Zheng Yang*ab
aKey Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: qzyang@mail.ipc.ac.cn; Fax: +86 10 62554670; Tel: +86 10 82543450
bCollege of Chemistry, Beijing Normal University, Beijing, 100875, P. R. China
cUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 5th December 2014
Abstract
We reported a BODIPY-based fluorescent probe for the simultaneous detection of GSH and Cys/Hcy. The nitrothiophenol moiety of the probe serves not only as a leaving group for the thiol-substitution reaction, but also a fluorescence quencher to provide a low emission background. The electron-withdrawing imidazolium group drastically increases reactivity of the Cys/Hcy-induced substitution–rearrangement reaction. The imidazolium group can further be replaced by GSH and resulted in a bithioether-product (λabs = 568 nm, λem = 588 nm), which showed distinct photophysical properties from the amino-product (λabs = 443 nm, λem = 530 nm) in the case of Cys/Hcy. It is noted that they exhibited great differences in absorption spectra of more than 120 nm. Thus, the simultaneous detection of GSH and Cys/Hcy can be achieved at different excitation wavelengths. The probe can quantitatively determinate the amount of Cys, Hcy and GSH in certain concentration ranges. We also found that the probe could detect GSH and Cys in living cells from different emission channels.
Introduction
Biothiols, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), play crucial roles in maintaining biological redox homeostasis in biological systems. Numerous investigations have demonstrated that abnormal levels of biothiols are associated with certain diseases.1 Consequently, the assessment of abnormal levels of thiol-containing substances in biological systems may provide evidence of the different functionality of thiols in biological processes, and further serve as significant factors for early diagnosis of some diseases. Fluorescent sensors are considered to be effective molecular tools that monitor and visualize intracellular analytes. Great effort has been devoted to develop reaction based fluorescent sensors for the detection of thiols in living systems.2 Most of the sensors can distinguish biothiols from other amino acids by taking advantage of the strong nucleophilic ability of thiols. However, the discriminations between biothiols with similar structures and reactivities are hampered. Given that levels of Cys, Hcy and GSH are related to different physiological processes and diseases, fluorescent probes that can discriminate between them have been of particular interest to scientists.Pioneered by Strongin group,3 the selective detection of Cys/Hcy over GSH could be achieved based on the cyclization of Cys/Hcy with aldehydes.4 They also reported the conjugate addition–cyclization reaction with acrylates,5 which inspired the simultaneous detection of Cys and Hcy.6 Some other methods, including the extended version of the reported strategies were also performed to selectively detection of Cys or Hcy.7 By contrast, the discrimination of GSH from Cys/Hcy has been investigated in less extent, such as the GSH-induced conjugate addition–cyclization reaction with acrylates or nucleophilic substitution of dinitrophenyl ester in CTAB,8 the electrostatic interactions between bis-spiropyran and GSH.9 In 2012, we reported a pioneering work that could produce a highly selective and ratiometric fluorescence change with GSH relative to Cys and Hcy.10 The thiol–halogen substitution reaction between chlorinated BODIPY and GSH produced sulfur-substituted BODIPY, which is distinct from the amino-substituted ones induced by the substitution–rearrangement reaction between chlorinated BODIPY and Cys/Hcy. It provided a new platform and inspired more extended work on the selective detection of biothiols.11 In order to explore the different functions of biothiols in physiological process, the simultaneous detection of different biothiols using one probe is highly valuable, but even more challenging. Some attractive examples reported by Strongin's and Guo's group are the simultaneous detection of Cys or/and Hcy and GSH from different emission channels.11d,11e,12 Although great advances have been made in the field of selective detection of biothiols, there are still some limitations in practical applications, such as long response time, the introduction of surfactant, etc. Herein we reported a BODIPY-based fluorescent probe for the selective and simultaneous detection of GSH and Cys/Hcy at different excitation wavelengths. The distinct excitation wavelengths would eliminate the interference from different analytes to the maximum extent. The probe showed turn-on fluorescence towards biothiols with extremely fast response, and could detect GSH and Cys in living cells from different emission channels.
Results and discussion
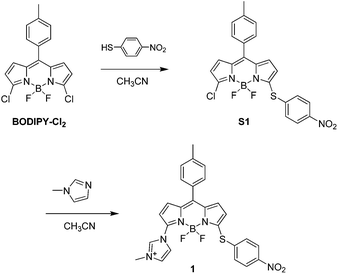
In our previous work, we reported the electron-rich substituents would impact on the reactivity of mono-chlorinated BODIPY towards nucleophilic aromatic substitution.10 The reported fluorescent probe showed a fast response to GSH. However, it would take an extended time to get rid of the interference from Hcy. In order to further elevate the reactivity, a more electron-withdrawing imidazolium group was introduced to the 3-position of BODIPY. In addition, the imidazolium salt would make the probe more hydrophilic for practical application in biological system. On the other hand, nitrothiophenol moiety with strong electron deficiency at 5-position of BODIPY served not only as a leaving group for the thiol-induced SNAr substitution reaction, but also as an electron acceptor to provide the photo-induced electron transfer (PET) process to ensure a low fluorescence background. We anticipated that this probe would yield thioether-product with GSH, which is distinct from the amino-product induced by substitution–rearrangement reaction with Cys/Hcy. Thus the simultaneous detection of GSH and Cys/Hcy could be achieved.By using 3,5-dichlorinated BODIPY as precursor, compound 1 was synthesized in two steps, as shown in Fig. 1. The structure of 1 was confirmed by 1H NMR, 13C NMR and HRMS spectra.
Initially we examined the absorption spectra of 1 in the presence of Cys, Hcy and GSH. As shown in Fig. 2, the free 1 exhibited a main absorption band centred at 528 nm. Upon addition of Cys/Hcy, a blue-shifted and widened absorption band centred at 443 nm emerged, which is ascribed to the typical amino-BODIPY. In the condition of GSH, the proposed product was expected to show a similar absorption band with 1. However, an obvious red-shifted absorption band was observed, unexpectedly. In the time-dependent absorption spectra (Fig. S1 in ESI†), upon addition of GSH, the absorption band centred at 528 nm decreased gradually, while a new absorption peak at 568 nm increased. Methyl mercaptoacetate, an organic soluble analogue of GSH, was used to study the reaction product. Upon treatment of 1 with methyl mercaptoacetate resulted in the disulfur-substituted BODIPY C1 (ESI†), which showed similar absorption spectrum with 1-GSH (Fig. S2 in ESI†). It indicated that not only nitrothiophenol, but also imidazolium group were replaced by GSH.
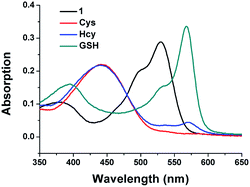 | ||
Fig. 2 Absorption spectra of 1 (10 μM) after 15 min addition of GSH, Cys and Hcy (1 mM) in acetonitrile/HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v, 20 mM, pH 7.4) at 37 °C. 99, v/v, 20 mM, pH 7.4) at 37 °C. | ||
Given that the reaction of 1 with Cys/Hcy and GSH exhibited distinct absorption spectra (Fig. 2), we performed the emission sensing behaviour of 1 in the presence of Cys, Hcy and GSH by using different excitation wavelength. As expected, the initial fluorescence of 1 was quite low, no matter under excited at 443 nm or 568 nm, due to the quenching effect of the nitro group through the PET process from BODIPY to nitrothiophenyl group.
Under the excitation at 443 nm, 1 showed an obvious emission enhancement at 530 nm upon addition of Cys or Hcy. In the case of GSH, due to the weak absorption at 443 nm, the emission spectra of 1 with addition of GSH induced almost no emission increase at 530 nm, but a weak fluorescence at 588 nm (Fig. 3a). By contrast, when excited at 568 nm, the reaction of 1 with GSH led to a dramatic fluorescence turn-on at 588 nm. Under the identical conditions, the addition of Cys caused almost no fluorescence changes. In the presence of Hcy, 1 showed a weak emission, which is relatively negligible compared to the strong fluorescence of GSH (Fig. 3b). The results indicated that 1 could simultaneously detect Cys/Hcy and GSH by using different excitations.
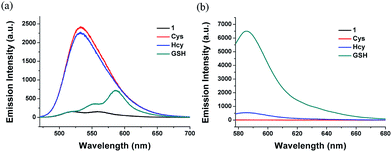 | ||
Fig. 3 Fluorescent spectra of 1 (10 μM) after 5 min addition of GSH, Cys and Hcy (1 mM) in acetonitrile/HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v, 20 mM, pH 7.4) at 37 °C. (a) λex = 443 nm. (b) λex = 568 nm. 99, v/v, 20 mM, pH 7.4) at 37 °C. (a) λex = 443 nm. (b) λex = 568 nm. | ||
The time-dependent emission spectra revealed the fast fluorescence responses of 1 towards biothiols. As shown in Fig. S3a,† under the excitation of 443 nm, with addition of Cys, the emission of 1 at 530 nm reached its maximum within seconds, suggesting the extremely fast kinetics of the amino-induced rearrangement reaction. For Hcy which would go through a six-membered transition state, the substitution–rearrangement reaction is complete within 2 min (Fig. S3a in ESI†). Noted the similar reaction in our former report11f would take several hours. It revealed that the electron-withdrawing imidazolium group did greatly increase the reactivity. In the case of GSH, a slight emission increase at the start was attributed to the fluorescence of monosulfur-substituted BODIPY. Then the fluorescence decreased gradually with the formation of final disulfur-substituted product. By contrast, under the excitation of 568 nm, the emission response at 588 nm to the addition of GSH showed a relatively slow kinetics probably due to the second step of sulfur substitution (Fig. S3b in ESI†). However, 5 min is long enough to distinguish GSH from Cys/Hcy (Fig. 3 and S3 in ESI†).
We measured the fluorescent changes under excitation of 528 nm to investigate the reaction process (Fig. 4 and S4 in ESI†). Upon addition of GSH to the solution of 1, an emission peak at 556 nm emerged at the very start. The emission at 556 nm is attributed to the fluorescence from an intermediate, whose nitrothiophenol group was replaced by GSH. However, the emission at 556 nm decreased quickly within a few minutes, while a new emission peak at 588 nm increased significantly. It indicated imidazolium group was also replaced by GSH through a second sulfur-substitution reaction (Fig. 4a). By contrast, upon addition of Cys, the emission spectra showed no emission enhancement at either around 530 nm or 588 nm (Fig. S4 in ESI†). It revealed that the first step reaction of 1 with Cys, that is the sulfur-substitution, could not be observed due to the extremely fast amino-induced intramolecular rearrangement reaction. For Hcy, an emission band at 556 nm was observed at the very beginning. Due to the relatively slower intramolecular-rearrangement reaction compared to Cys, a small amount of monosulfur-substituted products of 1 with Hcy went through the second sulfur-substitution reaction and resulted in a weak emission at 588 nm (Fig. 4b). The fluorescence enhancement at 588 nm for 1 with Hcy is neglectable compared with GSH under the identical conditions (Fig. 3 and S3 in ESI†), suggesting that Hcy would not disturb the detection of GSH. It also explained that Cys caused more fluorescence enhancement than Hcy.
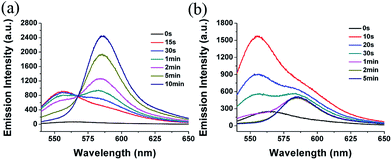 | ||
Fig. 4 Time-dependent fluorescence spectra of 1 (10 μM) with addition of (a) GSH (1 mM) and (b) Hcy (1 mM) in acetonitrile/HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v, 20 mM, pH 7.4) at 37 °C. λex = 528 nm. 99, v/v, 20 mM, pH 7.4) at 37 °C. λex = 528 nm. | ||
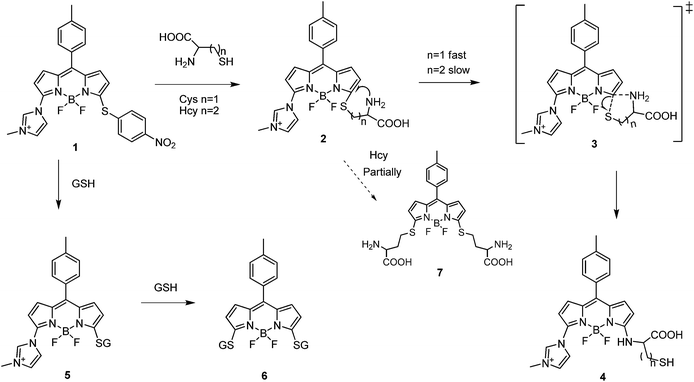
From the above observations, we proposed the reaction mechanism of 1 with Cys, Hcy and GSH, as illustrated in Fig. 5. The electron-withdrawing imidazolium group made 1 very active to the nucleophiles. The nitrothiophenol moiey was rapidly replaced by thiol group to produce 2 or 5. For GSH, the imidazolium group of 5 would be further replaced by the thiol group of GSH and resulted in the disulfur-substituted product 6. By contrast, for Cys and Hcy, Compound 2 would instantly go through an intramolecular rearrangement through a 5- or 6-membered transition state to yield 4. The amino substituent in 4 with strong electron-donating ability significantly decreased the reaction activity of nucleophilic substitution so that the imidazolium group could not be replaced by thiols anymore. However, the intramolecular rearrangement reaction for Hcy is relatively slower than Cys due to the less favourite 6-membered transition state, which allowed the slight formation of disulfur-substituted product 7. Theoretically, either of the amino groups in 7 could further replace thiol group, however, beyond the time scale of the experiment.
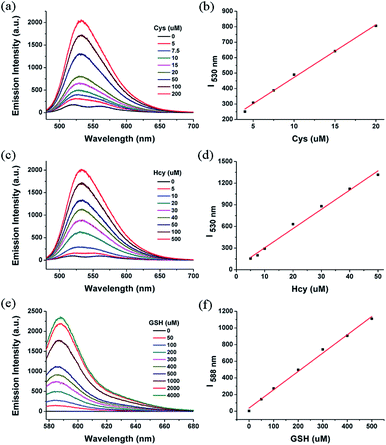
The quantitative determinations were measured with 1 by incremental addition of biothiols. The fluorescence intensity of 1 at 530 nm gradually increased according to the increasing amount of Cys or Hcy (Fig. 6a and c, S5 and S6 in ESI†). It is worth noting that the fluorescence almost remained unaffected when less than 0.4 equiv. of Cys or Hcy were added. The phenomena were in accordance with the reported literatures,11d,11e which was probably attributed to the formation of the bi-BODIPY through side reaction of 4 with unreacted 1. The emission intensity of 530 nm showed linear relationship with the Cys and Hcy concentrations ranging from 5 to 20 μM and 5 to 50 μM, respectively (Fig. 6b and d). For GSH, the emission at 588 nm increased significantly upon addition of increasing amount of GSH (Fig. 6e and S7 in ESI†), and the linearity range was 0–500 μM (Fig. 6f).
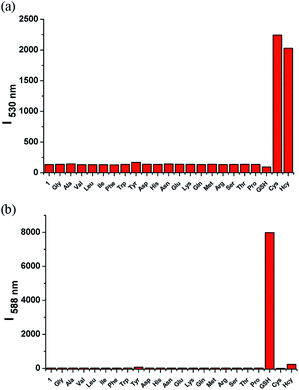
We evaluated the selectivity of 1 towards other physiologically relevant amino acids (e.g., Gly, Ala, Val, Leu, Ile, Phe, Trp, Tyr, Asp, His, Asn). With the excitation of 443 nm, only Cys/Hcy induced significant fluorescence enhancement at 530 nm. 1 exhibited almost no fluorescence intensity changes in the presence of other various amino acids. When excited at 568 nm, the addition of GSH caused significant fluorescence increase at 588 nm. The competitive amino acids induced almost no fluorescence intensity changes of 1. It suggested that 1 was highly selective for Cys/Hcy or GSH under different excitations (Fig. 7).
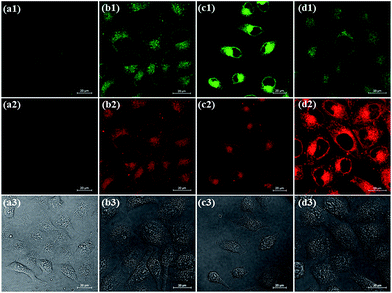
We further examined the capability of 1 to detect Cys and GSH in living cells (Fig. 8). HeLa cells were treated with N-ethylmaleimide (a thiol-blocking agent) prior to the addition of 1, and it showed almost no fluorescence in both green and red channels. By contrast, when HeLa cells were incubated with 1, the obvious fluorescence in both green and red channels was observed. It revealed 1 was responsive to intracellular thiols. When HeLa cells were pretreated with 0.5 mM Cys and then incubated with 1, a marked increase in green emission was observed. On the other hand, when HeLa cells were pretreated with 1 mM GSH and then incubated with 1, a distinct increase in green emission was observed. These results suggest that 1 could sense Cys and GSH from different emission channels in living cells.
Conclusions
In summary, we designed and synthesized a novel fluorescent probe 1 that could simultaneously detect Cys/Hcy and GSH under different excitations. 1 showed fast response to Cys/Hcy through substitution–rearrangement reaction and resulted in the amino-BODIPY. When excited at 443 nm, 1 exhibited significant fluorescence increase at 530 nm. By contrast, both of nitrothiophenol and imidazolium groups in 1 could be replaced by GSH, which led to the formation of disulfur-BODIPY. When excited at 568 nm, significant fluorescence enhancement of 1 at 588 nm was observed. 1 was applied to detect GSH and Cys in living cells from different emission channels. The interesting design strategy and reaction mechanism may inspire the design of new probes for discrimination of biothiols. The high selectivity of our probe to biothiols may give new insights for the exploration of selective detection of thiols in biological systems.Experimental section
Materials and instruments
All the reagents and solvents are of commercial quality and without further purification. 1H and 13C NMR spectra were recorded on an Advance Bruker 400 M spectrometer and referenced to solvent signals. Mass spectra were obtained on Bruker Apex IVFourier Transform Mass Spectrometer. Fluorescence spectra were determined on a Hitachi 4500 spectrophotometer. Absorption spectra were determined on a Shimadzu UV-1601PC UV-Visible spectrophotometer.Synthesis of probe 1
BODIPY–Cl2 (200 mg, 0.57 mmol) was dissolved in 50 mL acetonitrile and stirred at 0 °C. 4-Nitrothiophenol (43 mg, 0.28 mmol) in 20 mL acetonitrile was added slowly, and the mixture was stirred at 0 °C for 10 min. After evaporation, the residue was purified by column chromatography on silica gel (dichloromethane/petroleum ether = 1/1 as eluent) to give S1 (61 mg, 47%) as a purple-red solid. 1H NMR (400 MHz, CDCl3): δ 8.23 (d, 2H, J = 8.8 Hz), 7.70 (d, 2H, J = 8.8 Hz), 7.41 (d, 2H, J = 8.0 Hz), 7.33 (d, 2H, J = 8.0 Hz), 6.85 (m, 2H), 6.44 (d, 1H, J = 4.4 Hz), 6.21 (d, 1H, J = 4.4 Hz), 2.47 (s, 3H).Compound S1 (60 mg, 0.13 mmol) was dissolved in 20 mL acetonitrile. 1-Methylimidazole (53 mg, 0.65 mmol) was added to the solution. The mixture was stirred at r.t. for 3 h, and washed with water. The organic layer was collected and dried over NaSO4. After concentration, the residue was purified by column chromatography on silica gel (dichloromethane/methanol = 10/1 as eluent) to give compound 1 (45 mg, 67%) as a red solid. 1H NMR (400 MHz, CDCl3): δ 11.10 (s, 1H), 8.30 (d, 2H, J = 8.8 Hz), 8.06 (s, 1H), 7.81 (d, 2H, J = 8.8 Hz), 7.55 (s, 1H), 7.42 (d, 2H, J = 8.0 Hz), 7.36 (d, 2H, J = 8.0 Hz), 7.12 (d, 1H, J = 4.0 Hz), 7.01 (d, 1H, J = 4.4 Hz), 6.86 (d, 1H, J = 4.0 Hz), 6.13 (d, 1H, J = 4.4 Hz), 4.30 (s, 3H), 2.47 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 161.2, 149.0, 144.9, 142.4, 140.0, 139.0, 137.8, 137.4, 134.9, 134.4, 133.2, 130.7, 129.7, 128.7, 125.0, 123.5, 123.2, 121.9, 113.6, 37.4, 21.6. ESI-HRMS: calculated for M+ 516.14762, found 516.14709.
Cell culture and fluorescence imaging
HeLa cells were cultured in culture media (DMEM/F12 supplemented with 10% FBS, 50 unit per mL penicillin, and 50 μg mL−1 of streptomycin) at 37 °C under a humidified atmosphere containing 5% CO2 for 24 h. The cells were seeded in a 6-well plate at a density of 104 cells per well in culture media. The cells were incubated with 1 (2 μM) in culture media for 15 min, or pretreated with 1 mM N-ethylmaleimide (NEM), 500 μM Cys or 1 mM GSH in culture media for 15 min, washed with PBS, and further incubated with 1 (2 μM) in culture media for 15 min. Confocal fluorescence imaging was performed with Nikon A1R MP multiphoton microscopy with a 60× oil-immersion objective lens. Fluorescence was collected from different emission channels by 500–550 nm (excited at 488 nm) and 570–620 nm (excited at 561 nm) band pass filter.Acknowledgements
We thank Prof. Z. Niu and Dr M. Wu for providing HeLa cells. This work was financially supported by the 973 program (2013CB933800), National Natural Science Foundation of China (21222210, 21402216, 21102155).Notes and references
- (a) S. Shahrokhian, Anal. Chem., 2001, 73, 5972 CrossRef CAS; (b) S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'Agostino, P. W. F. Wilson and P. A. Wolf, N. Engl. J. Med., 2002, 346, 476 CrossRef CAS PubMed; (c) D. M. Townsend, K. D. Tew and H. Tapiero, Biomed. Pharmacother., 2003, 57, 145 CrossRef CAS.
- (a) H. S. Jung, X. Chen, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019 RSC; (b) H. Peng, W. Chen, Y. Cheng, L. Hakuna, R. Strongin and B. Wang, Sensors, 2012, 12, 15907 CrossRef CAS PubMed; (c) X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120 RSC; (d) K. Xu, M. Qiang, W. Gao, R. Su, N. Li, Y. Gao, Y. Xie, F. Kong and B. Tang, Chem. Sci., 2013, 4, 1079 RSC; (e) W. Sun, J. Fan, C. Hu, J. Cao, H. Zhang, X. Xiong, J. Wang, S. Cui, S. Sun and X. Peng, Chem. Commun., 2013, 49, 3890 RSC; (f) B. K. McMahon and T. Gunnlaugsson, J. Am. Chem. Soc., 2012, 134, 10725 CrossRef CAS PubMed; (g) L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510 CrossRef CAS PubMed; (h) R. Wang, L. Chen, P. Liu, Q. Zhang and Y. Wang, Chem.–Eur. J., 2012, 18, 11343 CrossRef CAS PubMed; (i) Y.-Q. Sun, M. Chen, J. Liu, X. Lv, J. Li and W. Guo, Chem. Commun., 2011, 47, 11029 RSC; (j) B. Tang, Y. Xing, P. Li, N. Zhang, F. Yu and G. Yang, J. Am. Chem. Soc., 2007, 129, 11666 CrossRef CAS PubMed.
- (a) O. Rusin, N. N. St. Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 438 CrossRef CAS PubMed; (b) W. Wang, O. Rusin, X. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127, 15949 CrossRef CAS PubMed.
- (a) F. Kong, R. Liu, R. Chu, X. Wang, K. Xu and B. Tang, Chem. Commun., 2013, 49, 9176 RSC; (b) P. Wang, J. Liu, X. Lv, Y. Liu, Y. Zhao and W. Guo, Org. Lett., 2012, 14, 520 CrossRef CAS PubMed; (c) M. Hu, J. Fan, H. Li, K. Song, S. Wang, G. Cheng and X. Peng, Org. Biomol. Chem., 2011, 9, 980 RSC; (d) S. Lim, J. O. Escobedo, M. Lowry, X. Xu and R. Strongin, Chem. Commun., 2010, 46, 5707 RSC; (e) L. Xiong, Q. Zhao, H. Chen, Y. Wu, Z. Dong, Z. Zhou and F. Li, Inorg. Chem., 2010, 49, 6402 CrossRef CAS PubMed; (f) W. Lin, L. Long, L. Yuan, Z. Cao, B. Chen and W. Tan, Org. Lett., 2008, 10, 5577 CrossRef CAS PubMed.
- X. Yang, Y. Guo and R. M. Strongin, Angew. Chem., Int. Ed., 2011, 50, 10690 CrossRef CAS PubMed.
- (a) Z. Guo, S. Nam, S. Park and J. Yoon, Chem. Sci., 2012, 3, 2760 RSC; (b) H. Wang, G. Zhou, H. Gai and X. Chen, Chem. Commun., 2012, 48, 8341 RSC.
- (a) H. Y. Lee, Y. P. Choi, S. Kim, T. Yoon, Z. Guo, S. Lee, K. M. K. Swamy, G. Kim, J. Y. Lee, I. Shin and J. Yoon, Chem. Commun., 2014, 50, 6967 RSC; (b) X. Zhou, X. Jin, G. Sun and X. Wu, Chem.–Eur. J., 2013, 19, 7817 CrossRef CAS PubMed; (c) M. Zhang, Y. Wu, S. Zhang, H. Zhu, Q. Wu, L. Jiao and E. Hao, Chem. Commun., 2012, 48, 8925 RSC; (d) L. Yuan, W. Lin and Y. Yang, Chem. Commun., 2011, 47, 6275 RSC; (e) H. Li, J. Fan, J. Wang, M. Tian, J. Du, S. Sun, P. Sun and X. Peng, Chem. Commun., 2009, 5904 RSC; (f) M. Zhang, M. Yu, F. Li, M. Zhu, M. Li, Y. Gao, L. Li, Z. Liu, J. Zhang, D. Zhang, T. Yi and C. Huang, J. Am. Chem. Soc., 2007, 129, 10322 CrossRef CAS PubMed.
- (a) Y. Guo, X. Yang, L. Hakuna, A. Barve, J. O. Escobedo, M. Lowry and R. M. Strongin, Sensors, 2012, 12, 5940 CrossRef CAS PubMed; (b) L. Wang, H. Chen, H. Wang, F. Wang, S. Kambam, Y. Wang, W. Zhao and X. Chen, Sens. Actuators, B, 2014, 192, 708 CrossRef CAS PubMed.
- N. Shao, J. Jin, H. Wang, J. Zheng, R. Yang, W. Chan and Z. Abliz, J. Am. Chem. Soc., 2010, 132, 725 CrossRef CAS PubMed.
- L.-Y. Niu, Y.-S. Guan, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928 CrossRef CAS PubMed.
- (a) S.-Y. Lim, K.-H. Hong, D. I. Kim, H. Kwon and H.-J. Kim, J. Am. Chem. Soc., 2014, 136, 7018 CrossRef CAS PubMed; (b) L.-Y. Niu, H.-R. Zheng, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Analyst, 2014, 139, 1389 RSC; (c) Y.-S. Guan, L.-Y. Niu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, RSC Adv., 2014, 4, 8360 RSC; (d) J. Liu, Y.-Q. Sun, H. Zhang, Y. Huo, Y. Shi and W. Guo, Chem. Sci., 2014, 5, 3183 RSC; (e) J. Liu, Y.-Q. Sun, Y. Huo, H. Zhang, L. Wang, P. Zhang, D. Song, Y. Shi and W. Guo, J. Am. Chem. Soc., 2013, 136, 574 CrossRef PubMed; (f) L.-Y. Niu, Y.-S. Guan, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Commun., 2013, 49, 1294 RSC.
- (a) X.-F. Yang, Q. Huang, Y. Zhong, Z. Li, H. Li, M. Lowry, J. O. Escobedo and R. M. Strongin, Chem. Sci., 2014, 5, 2177 RSC; (b) F. Wang, Z. Guo, X. Li, X. Li and C. Zhao, Chem.–Eur. J., 2014, 20, 11471 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of compounds C1; time-dependent absorption spectra of 1 in addition of GSH; time-dependent emission intensity changes of 1 upon addition of Cys, Hcy and GSH, etc. See DOI: 10.1039/c4ra13526a |
| This journal is © The Royal Society of Chemistry 2015 |