Synergistic mechanism between laurel alkanolamide and hydrophobically associating polyacrylamide in solutions with high salinity†
Quanhua Denga,
Haiping Lib,
Xulong Caoc,
Yong Yangc,
Xinwang Songc and
Ying Li*a
aKey Laboratory for Colloid and Interface Chemistry of Education Ministry, Shandong University, Jinan 250100, P.R. China. E-mail: yingli@sdu.edu.cn; Fax: +86-531-88362078; Tel: +86-531-88362078
bNational Engineering Technology Research Center for Colloidal Materials, Shandong University, Jinan 250100, P.R. China
cGeological Scientific Research Institute, Shengli Oilfield, Dongying 257015, P.R. China
First published on 19th January 2015
Abstract
Synergism of water-soluble hydrophobically associating polyacrylamide (HA-PAM) with nonionic surfactant laurel alkanolamide (LAA) was investigated via the rheology, fluorescence spectroscopy and dissipative particle dynamics (DPD) simulation methods. The viscosity and elasticity of HA-PAM solutions increased in a large LAA concentration range, which was principally ascribed to the crosslinking effect of LAA by aggregating to the hydrophobic chains of HA-PAM molecules as confirmed by the DPD simulation and experimental results. The crosslinking effect was enhanced in the presence of an appropriate amount of electrolyte or with increasing temperature in the studied temperature range of 20–70 °C. Thus, the LAA not only significantly enhanced the salt resistance of HA-PAM but also retarded the decrease of the viscosity and elasticity of the HA-PAM solutions at high temperature. The HA-PAM/LAA binary systems exhibit great potential for application in tertiary oil recovery of oil fields with high salinity.
1. Introduction
Polymer and polymer/surfactant flooding systems have been extensively used in enhanced oil recovery (EOR),1 in which polymers are used as thickeners to increase the viscosity of the flooding system and adjust the mobility ratio of the water and oil phases. Polymers are indispensable for the high oil displacement efficiency of the flooding systems. Partially hydrolyzed polyacrylamide (HPAM)2 is the most widely used polymer in EOR, but the poor salt and shear resistance of HPAM limit its application in reservoirs with high salinity.In recent years, polymers containing salt resistance components such as amphoteric or nonionic side groups3,4 have been reported with strong salt and shear resistance. More exciting progress comes from the advance of the comb-shaped,5 hydrophobically associating6 polymers, which could form reversible networks, resulting in a dramatic increase in apparent viscosity even at a low concentration or under high salinity, and attracted the most interest of researchers. According to the literature reports,7 hydrophobically associating polyacrylamide (HA-PAM) exhibited much better salt resistance than HPAM and have showed great potential for application in reservoirs with high salinity.
In most of the actual practices, EOR for example, polymers were used, combined with surfactant. The interaction between surfactants and polymers was an interesting topic, attracting much more attention for a long time. Surfactants have strong influences on the rheological properties8 and salt resistance of polymeric solutions.9–11 The polymer/surfactant systems with opposite charge12–15 have been paid much attention. The viscosity of the solution could be greatly improved because of the inter- or intra-molecular cross-links driven by the electrostatic attracting interactions. But the synergistic effect is very sensitive to the concentration ratio of the surfactants and polymers, and these mixed solutions always exhibit a bad salt resistance, because the electrostatic interactions are sensitive to electrolytes, and minor electrolytes lead to a sharp decrease of the viscosity of the solutions.16 The combination of the polyelectrolytes and ionic surfactants with the same charge or nonionic surfactants were also reported. Hydrophobic interaction was the driving force to enhance the formation of molecular network, and some complex exhibited better salt resistance.11,17 The synergetic effect between HA-PAM and surfactants is still unclear, and the related reports were rare. To seek appropriate surfactants to cooperate with HA-PAM for further enhancing its salt resistance and oil displacement capability is desired in practical areas.
In this study, the rheological properties of mixed solutions of HA-PAM and anionic, amphoteric and nonionic surfactants were investigated. The salt resistance of HA-PAM solutions was greatly enhanced in the presence of nonionic surfactant laurel alkanolamide (LAA). The synergism between HA-PAM and LAA were investigated by combining experimental and molecular simulation methods. The HA-PAM/LAA (H/L) binary system exhibits high thickening capacity in large concentration ratio or under high salinity. This system has great potential for application as a polymer/surfactant flooding system in high salinity reservoirs.
2. Materials and methods
2.1. Materials
HA-PAM with a viscosity-averaged molecular weight of 3.6 × 105 and cocamidopropyl betaine (CAB, 30% aqueous solution, 99% purity) were provided by Geological Scientific Research Institute of Shengli oil field (Sinopec, China).7 Laurel alkanolamide (LAA) was synthesized in our lab and purified according to ref. 18.18 Sodium dodecyl sulfate (SDS, 99% purity) was obtained from Cxbio Biotechnology Co. Ltd. (China). Dodecyl sulphobetaine (DSB, analytical pure) was synthesized and purified by Dr J. Chen in Jin Ling Petrochemical Co. (Sinopec, China). Nonaethylene glycol monododecyl ether (C12E9, 99% purity) was purchased from Sigma-Aldrich. Deionized water was used in all the experiments. Chemical structures of HA-PAM and LAA are shown in Fig. 1. Structures of the other surfactants are shown in Fig. S1.†The HA-PAM and LAA stock solutions with concentrations of 0.2% and 1% were prepared by dissolving 0.2 g HA-PAM and 1.0 g LAA in 99.8 and 99.0 g deionized water, respectively. Solutions with low concentrations of HA-PAM and/or LAA were obtained via the dilution of the stock solutions. The pH values of all the solutions are 7.28 ± 0.19. The NaCl concentrations of solutions were adjusted by adding 10 or 1% NaCl bulk solutions. The HA-PAM concentration is 0.1% in all the related solutions in this paper unless special explanation. HA-PAM solutions containing pyrene (about 3.03 × 10−4 g L−l) were prepared by adding 20 μL of 0.15 g L−l pyrene ethanol solution to 10 mL of 0.1% HA-PAM aqueous solution.
2.2. Rheological measurement
Rheological measurements were carried out on a Haake RS75 Rheometer (Germany) with a Z41 Ti coaxial cylinder sensor system. All of the samples were rested for more than 12 h to eliminate the bubbles before measurement.In the steady-state shearing experiment, the viscosity was measured at the shear rates (![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) ) ranging from 0 to 1000 s−1 with a check gradient of 0.5 (Δτ/τ)/Δt% where τ and t is the shear stress and time, respectively, and the maximum waiting time for each shear rate step was 20 s. The frequency sweep measurements were carried out at the angular frequency (ω) of 0.05–100 rad s−1 and stress of 0.04 Pa (in the linear viscoelastic region). The equilibrium values of dynamic viscosity (|η*|) and moduli (G′ and G′′) were measured at ω = 0.2 rad s−1 in the linear viscoelastic region. The experiment temperature was controlled to be 25.0 ± 0.1 °C, unless the temperature effect was investigated.
) ranging from 0 to 1000 s−1 with a check gradient of 0.5 (Δτ/τ)/Δt% where τ and t is the shear stress and time, respectively, and the maximum waiting time for each shear rate step was 20 s. The frequency sweep measurements were carried out at the angular frequency (ω) of 0.05–100 rad s−1 and stress of 0.04 Pa (in the linear viscoelastic region). The equilibrium values of dynamic viscosity (|η*|) and moduli (G′ and G′′) were measured at ω = 0.2 rad s−1 in the linear viscoelastic region. The experiment temperature was controlled to be 25.0 ± 0.1 °C, unless the temperature effect was investigated.
The “gain in viscosity”,19 Sη is defined to denote the interacting strength between HA-PAM and LAA:
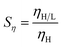 | (1) |
2.3. Fluorescence and surface tension measurements
Fluorescence spectra of solutions were detected with a Perkin-Elmer LS-55 spectrofluorometer using a quartz cuvette (1.0 cm × 1.0 cm). The excitation and emission slit widths were set at 7.5 nm. The emission spectra were recorded from 350 to 650 nm with excitation wavelength fixed at 335 nm. The fluorescence intensity ratio at 373 and 384 nm, namely, I1/I3 served as a parameter representing the micropolarity of aggregates. Pyrene was used as a fluorescent probe with the concentration of 3.03 × 10−4 g L−l.Surface tension measurements of various concentrations of LAA solutions were performed on a K100 Processor Tensiometer (Krüss Co., Germany) using the Wilhelmy ring at 30.0 ± 0.1 °C. Before each measurement, the plate was carefully cleaned with deionized water and flamed. The surface tension of deionized water was measured to calibrate the tensiometer and to check the cleanliness of the sample pool.
2.4. DPD simulation
Simulations were carried out with the DPD package from Accelrys (version 5.0).20 A cubic cell of size 20 × 20 × 20 Rc3 was built, where Rc = 9.5 was the cut-off radius. 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 time steps per simulation were set to assure the system attained dynamic equilibrium. The number density and the spring constant between different beads were 3.0 and 4.0, respectively. The temperature was 1.0 kBT according to Groot's work.21 Flory–Huggins parameters (χij) were obtained in Blend module. They could be converted into the interaction parameters (aij) by the equation, aij = χij × 3.27 + aii. Table 1 shows the interaction parameters aij used in this work. The diffusion coefficient of a DPD particle was interpreted as the ratio of the distance that fluid particles diffused through to the time they took.20 Larger diffusion coefficient of water molecules meant lower viscosity of solutions.
000 time steps per simulation were set to assure the system attained dynamic equilibrium. The number density and the spring constant between different beads were 3.0 and 4.0, respectively. The temperature was 1.0 kBT according to Groot's work.21 Flory–Huggins parameters (χij) were obtained in Blend module. They could be converted into the interaction parameters (aij) by the equation, aij = χij × 3.27 + aii. Table 1 shows the interaction parameters aij used in this work. The diffusion coefficient of a DPD particle was interpreted as the ratio of the distance that fluid particles diffused through to the time they took.20 Larger diffusion coefficient of water molecules meant lower viscosity of solutions.
| Bead | a | b | c | d | e | f | h | t | w |
|---|---|---|---|---|---|---|---|---|---|
| a | 80 | ||||||||
| b | 22.9 | 25 | |||||||
| c | 14 | 29.8 | 70 | ||||||
| d | 75.4 | 79.1 | 47 | 25 | |||||
| e | 29 | 29.5 | 27.1 | 70.9 | 25 | ||||
| f | 90 | 45 | 16 | 73.3 | 55.3 | 100 | |||
| h | 20 | 23.7 | 29.5 | 49.4 | 27.5 | 27.4 | 25 | ||
| t | 51.7 | 55.9 | 32.3 | 33.1 | 53.5 | 66.1 | 36.2 | 25 | |
| w | 15 | 27 | 28 | 64.4 | 31.5 | 9.6 | 30.7 | 56.5 | 25 |
3. Results and discussion
3.1. Rheological properties of the mixed solutions of HA-PAM and various surfactants
Fig. 2 shows the variation of the viscosity of 0.1% HA-PAM solutions with concentrations of CAB, DSB, LAA, C12E9 and SDS. The critical micelle concentrations of these surfactants are 0.045, 0.072, 0.010 (Fig. S2†), 0.0058 (ref. 22) and 0.23%,23 respectively. C12E9 exhibits little effect on the viscosity of HA-PAM solutions. The ionic surfactants (CAB, DSB and SDS) all induce a slight increase, followed by a decrease of HA-PAM solution viscosity with increasing surfactant concentrations. The slight increase of the viscosity results from the formation of surfactant micelle bridges between different polymer chains. Compared with the nonionic surfactant C12E9, the ionic surfactants induce a more abrupt viscosity decrease of HA-PAM solutions at the surfactant concentration higher than 0.1%, which might be because of the increase of inter-molecular electrostatic repulsion induced by the co-aggregation of ionic surfactant and the screening effect of excess counter ions.24–26 Being different from the above systems, the HA-PAM/LAA (H/L) combined system exhibits the strongest thickening ability at the concentration (Ca) less than 0.08%. Two viscosity maximums are observed at Ca = 0.008 and 0.02%, respectively.3.2. Effects of LAA concentration Ca and ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/h3_i_char_e0a2.gif) on the rheological properties of H/L solutions
on the rheological properties of H/L solutions
Fig. 3 shows the dynamic and steady viscosity of H/L solutions as a function of ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) and Ca. Similar viscosity variation trend with increasing Ca are observed at various
and Ca. Similar viscosity variation trend with increasing Ca are observed at various ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) (Fig. 2 and 3), but the first viscosity maximum is less obvious under the steady shear than the oscillation shear (Fig. 2 and 3). DPD simulation results of the H/L solutions also show two minimums of the diffusion coefficient of water molecules in the systems with increasing alkanolamide concentration in the simulation (Ca (in simulation)) (Fig. 4), which agreed very well with the experiment results, indicating that the bead–bead interaction parameters aij used in the simulation are reasonable.
(Fig. 2 and 3), but the first viscosity maximum is less obvious under the steady shear than the oscillation shear (Fig. 2 and 3). DPD simulation results of the H/L solutions also show two minimums of the diffusion coefficient of water molecules in the systems with increasing alkanolamide concentration in the simulation (Ca (in simulation)) (Fig. 4), which agreed very well with the experiment results, indicating that the bead–bead interaction parameters aij used in the simulation are reasonable.
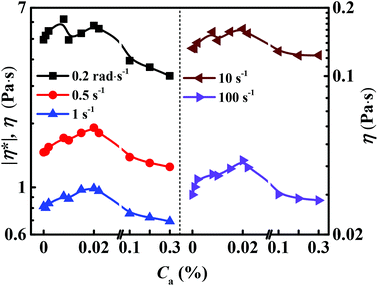 | ||
| Fig. 3 Effect of LAA concentration (Ca) on dynamic and steady viscosity of HA-PAM solutions at different shear rates. | ||
 | ||
| Fig. 4 Water diffusivity in the aqueous system as a function of LAA concentration at a shear rate of 0.005 in the simulation. | ||
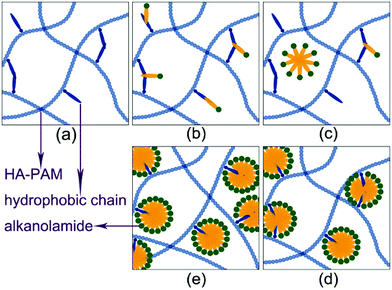
According to simulation results shown in Fig. 5, the hydrophobic side chain of the HA-PAM molecules cluster to form microdomains (Fig. 5b). When LAA was added to the HA-PAM solutions, the LAA molecules aggregate around these hydrophobic domains (Fig. 5c), and mixed micelle-like associations containing LAA molecules and hydrophobic segments of different HA-PAM molecules were formed. Herein, the LAA acts as crosslinkers which contributes to the increase of the intermolecular hydrophobic interaction, resulting in the viscosity increase of mixed solutions (Fig. 3). Fig. S3† shows the fluorescence spectra of the H/L solutions at different Ca. The increase of I3/I1 values with increasing Ca also means that more hydrophobic microdomains were formed.
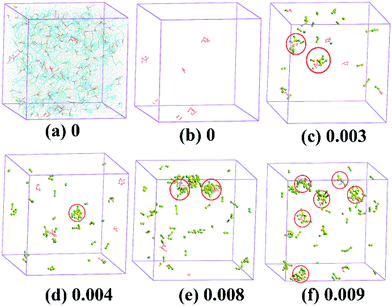 | ||
| Fig. 5 Configurations of H/L at shear rate of 0.005 and various LAA concentrations: (a) 0 with all the beads; (b) 0; (c) 0.003; (d) 0.004; (e) 0.008; (f) 0.009. Colors of the beads are the same as those in Fig. 1: (a) (sodium acrylate, golden yellow); (b) (acrylamide, light blue); (c) and (d) (hydrophobically associating monomer, orange and red); and (e) and (f) (sodium 2-acrylamido-2-methylpropanesulfonate, brown and pink); h and t (surfactant head and tail, green and yellow) in the simulation. | ||
When the LAA concentration increases, independent LAA micelles were found in the bulk phase (Fig. 5d), the desorption of LAA from the hydrophobic microdomains of the polymer molecules may lead to the decrease of the viscosity of the mixed solutions, as shown in Fig. 3. As the LAA concentration increases further, independent micelle were formed around the hydrophobic side chains of the HA-PAM (Fig. 5f).27–29 The intermolecular association of HA-PAM molecules induced by coaggregation of the hydrophobic side groups30 would be broken, leading to the viscosity decrease of the mixed solutions, corresponding well with the experimental results in Fig. 3.
By comparing the experimental and simulation results, we conclude that the LAA molecules aggregate together with the hydrophobic side chain of the HA-PAM molecule, and the simulation results show that the formed aggregates containing LAA micelles and hydrophobic segments of HA-PAM molecules (Fig. 5e) behave like crosslinkers to bind the HA-PAM molecules together and strengthen the network structure, leading to an increase of the viscosity of the bulk solutions (Fig. 3).27–29 The whole process of the effect of the LAA concentration on the micromolecular behavior and the properties of the H/L mixed systems were shown in Fig. 6. At Ca < 0.008%, the LAA molecules get clustered with the hydrophobic segments of HA-PAM (Fig. 6b). At 0.008% < Ca < 0.01%, the LAA molecules tend to form micelles in bulk solution (Fig. 6c). At 0.01% < Ca < 0.02%, the micelle-like associations containing LAA molecules and hydrophobic segments from different HA-PAM molecules were formed (Fig. 6d), and at Ca > 0.02%, independent micelles were formed around each hydrophobic side chains of the HA-PAM (Fig. 6e).27–29 The intermolecular association of HA-PAM molecules induced by coaggregation of the hydrophobic side groups30 would be broken, leading to the decrease of the viscosity of the mixed solutions. The increasing I3/I1 value with Ca in Fig. S3† indicates the increase of the amount of hydrophobic microdomains in solutions, corresponding to the variation of the microstructure shown in Fig. 5 and 6.
In the EOR area, not only the viscosity but also the elasticity of the flooding solutions are important in the oil displacement process.31 The frequency scan curves of H/L solutions at Ca = 0.02% are shown in Fig. 7a. The G′ are higher than G′′ in the studied ω range, indicating the dominant elastic behavior of the solution. Fig. 7b shows the modulus ratios (SG) of the H/L mixed solutions to HA-PAM solutions. Both SG′ and SG′′ are larger than 1, and the phase angle tangent values of the H/L mixed solutions are less than those of the HA-PAM solutions (Fig. S4†), indicating the hydrophobic association interaction between HA-PAM and LAA also induces the enhancement of the elasticity of the solutions.
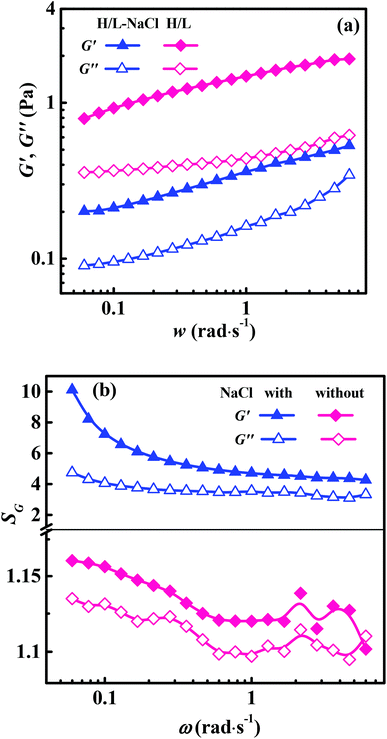 | ||
| Fig. 7 (a) Frequency scan curves for H/L solutions containing 0.02% LAA and (b) modulus ratios (SG) of H/L to HA-PAM solutions7 with or without 0.2% NaCl. | ||
The coaggregation of the surfactants and hydrophobic side chains of the HA-PAM plays the key role in formation of the network to maintain the high viscosity and elasticity of the mixed solutions. Being different from the H/L mixed system, the viscosity of the mixed solutions of C12E9 and HA-PAM changed slightly, as shown in Fig. 2. The HLB of C12E9 was higher than LAA, and the driven force for hydrophobic association is weaker for C12E9 comparing with LAA. It is concluded that suitable hydrophilic–lipophilic balance is needed for the surfactants to get coaggregated beside the hydrophobic side chains of the HA-PAM.
3.3. Effect of NaCl on the rheological properties of H/L solutions
Fig. 8a shows the variation of dynamic and steady viscosity of H/L solutions containing 0.02% LAA as a function of the NaCl concentration (CNaCl). With increasing CNaCl, the viscosity of the H/L solutions exhibits the same variation trend as the HA-PAM solutions.7 But for the H/L solutions, a prominent viscosity enhancement is observed in the presence of NaCl at![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 0.5–100 s−1 (Fig. S5†). The Sη values between HA-PAM and LAA at different CNaCl are shown in Fig. 8b. With increasing CNaCl, Sη increases first and then decreases, with a maximum observed at about CNaCl = 0.2% where the H/L solutions exhibit the largest viscosity (Fig. 8a). Sη can decrease to be less than 1 only at very high CNaCl. So the H/L binary system has better salt-tolerance than HA-PAM.
= 0.5–100 s−1 (Fig. S5†). The Sη values between HA-PAM and LAA at different CNaCl are shown in Fig. 8b. With increasing CNaCl, Sη increases first and then decreases, with a maximum observed at about CNaCl = 0.2% where the H/L solutions exhibit the largest viscosity (Fig. 8a). Sη can decrease to be less than 1 only at very high CNaCl. So the H/L binary system has better salt-tolerance than HA-PAM.
 | ||
| Fig. 8 (a) Dynamic (■) and steady viscosity of H/L solutions containing 0.1% HA-PAM and 0.02% LAA and (b) interaction strength (Sη) of HA-PAM with LAA at various NaCl concentrations and shear rates. | ||
The addition of NaCl enhanced the hydrophobic interaction between hydrophobic chains of HA-PAM and LAA (Fig. 5e).32 With increasing NaCl concentration, the salting-out effect disturbs the hydration of the polymer and surfactant, and thereby promotes the hydrophobic association,33 so Sη increases continuously with the increase of CNaCl at CNaCl < 0.2%. Meanwhile, apart from the hydrophobic units of the HA-PAM, there are several charged groups such as COO−, SO3−, and N+ in the molecules. The screening effect of NaCl can decrease the intramolecular electrostatic repulsion caused by these charged groups, leading to the curling of the polymer molecules.34,35 The molecular curling enhances the intramolecular and impairs the intermolecular hydrophobic interactions between HA-PAM molecules,35 and simultaneously decreases the hydrophobic interaction between HA-PAM and LAA molecules, which makes the viscosity of H/L solutions decrease slightly at CNaCl < 0.02% (Fig. 8a), and Sη decrease significantly with increasing CNaCl at CNaCl > 0.2%. Sη < 1 at higher CNaCl is probably because the LAA participates in the intramolecular hydrophobic interaction of HA-PAM molecules and increases the curling of HA-PAM molecules.
The ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) also has a great effect on the viscosity of H/L solutions and Sη. With increasing
also has a great effect on the viscosity of H/L solutions and Sη. With increasing ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) , the steady viscosity of the solutions gradually decreases (Fig. 8a). At CNaCl < 0.04%, the |η*| of the solutions is larger than the steady viscosity at
, the steady viscosity of the solutions gradually decreases (Fig. 8a). At CNaCl < 0.04%, the |η*| of the solutions is larger than the steady viscosity at ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 0.5–100 s−1, while at CNaCl > 0.04%, the steady viscosity at
= 0.5–100 s−1, while at CNaCl > 0.04%, the steady viscosity at ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 0.5 s−1 is larger than the |η*| of the solutions, which shows that the addition of NaCl enhances the shear-tolerance of the system. The shear could induce the variation of the molecular conformation and coaggregation behavior. On one hand, the shear can destroy the inter/intra-molecular hydrophobic interactions.36 On the other hand, the shear can inhibit the macromolecular curl. At low CNaCl, the intramolecular hydrophobic interaction in the H/L system is weak, so the shear principally destroy the intermolecular hydrophobic interaction, which leads to the decrease of solution viscosity. At high CNaCl, the screening effect of NaCl induces the severe curl of HA-PAM molecules, as discussed above. The shear mainly destroys the intramolecular hydrophobic interaction and dissociates the LAA molecules from the intramolecular hydrophobic microdomains, which makes the steady viscosities of solutions at
= 0.5 s−1 is larger than the |η*| of the solutions, which shows that the addition of NaCl enhances the shear-tolerance of the system. The shear could induce the variation of the molecular conformation and coaggregation behavior. On one hand, the shear can destroy the inter/intra-molecular hydrophobic interactions.36 On the other hand, the shear can inhibit the macromolecular curl. At low CNaCl, the intramolecular hydrophobic interaction in the H/L system is weak, so the shear principally destroy the intermolecular hydrophobic interaction, which leads to the decrease of solution viscosity. At high CNaCl, the screening effect of NaCl induces the severe curl of HA-PAM molecules, as discussed above. The shear mainly destroys the intramolecular hydrophobic interaction and dissociates the LAA molecules from the intramolecular hydrophobic microdomains, which makes the steady viscosities of solutions at ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = 0.5 s−1 higher than the |η*|.
= 0.5 s−1 higher than the |η*|.
The frequency scan curves of H/L solutions with Ca = 0.02% and CNaCl = 0.2% and SG values are shown in Fig. 7. G′ > G′′ indicates the solution exhibits a dominant elastic behavior. The SG′ and SG′′ are larger than 1, and the phase angle tangent values of the H/L solutions are less than those of the HA-PAM solutions (Fig. S4†), indicating the hydrophobic association between HA-PAM and LAA increases the elasticity of solutions. The SG′ and SG′′ values of solutions with NaCl are much larger than those without NaCl (Fig. 7b), which reveals that in the presence of NaCl, the elasticity enhancement of solutions induced by the hydrophobic interaction between HA-PAM and LAA is more prominent.
Overall, the LAA contributes to the viscosity and elasticity enhancement of HA-PAM solutions at CNaCl < 1%, indicating the salt tolerance of H/L solutions is better than that of the HA-PAM solutions.
3.4. Effect of temperature on rheological properties of H/L solutions
Temperature (T) can substantially influence the rheological properties of the hydrophobic associating polymer solutions.37 Fig. 9a shows the variation of |η*|, G′ and G′′ with T for the H/L solutions with Ca = 0.02%. G′ is always higher than G′′ in the tested temperature range for all solutions, indicating a dominant elastic behavior. With increasing T, the |η*| and G′ of H/L solutions exhibit the similar trend as the HA-PAM solutions,7 i.e. gradual decrease, except a plateau appears at T = 50–60 °C, respectively. The T has little effect on the G′′ of solutions. The decrease of |η*|and G′ of solutions with increasing T is because high temperature accelerates the macromolecular movement, thins the hydrated layer and destroys the intermolecular interaction.38 The appearance of plateaus reveals the decrease of |η*| and G′ is retarded (Fig. S6†), further indicating that the hydrophobic interaction between HA-PAM and LAA contributes to the viscosity enhancement of solutions. | ||
| Fig. 9 Effect of temperature on (a) dynamic viscosity and moduli of H/L solutions containing 0.02% LAA, and (b) viscosity and modulus ratios (Sη and SG) of H/L to HA-PAM solutions. | ||
In order to assess the effect of T on the interaction strength of HA-PAM with LAA, Sη and SG of solutions are shown in Fig. 9b. In the studied T range, the Sη and SG are always larger than 1, indicating the interaction between HA-PAM and LAA leads to a viscosity increase. With increasing T, both Sη and SG first increase and then decrease with a maximum observed at T = 60 °C. As is reported, the hydrophobic association is an endothermic process,38,39 and the increase of temperature is favorable for hydrophobic interaction enhancement. Thus, The Sη and SG increase with T at T < 60 °C. The reduction of Sη and SG at T > 60 °C is caused by the strong thermal motion of the molecules which destroys the interaction between HA-PAM and LAA.
In the presence of NaCl, the effects of T on the rheological properties of H/L solutions, Sη and SG are different from the results without NaCl. As shown in Fig. 10a and S7,† at CNaCl = 0.2%, the |η*|, G′ and G′′ decrease more prominently with increasing T than those without NaCl (Fig. 9a and S6†), and no plateau is observed, which is similar with those for the pure HA-PAM solutions.7 But G′ is always larger than G′′ in the tested temperature range for the H/L solutions, indicating a dominant elastic behavior. While for the HA-PAM solutions, G′ is larger than G′′ at T = 20–35 °C and less than G′′ at T = 35–70 °C. Fig. 10b shows the changes of Sη and SG with T. All Sη, SG′ and SG′′ gradually increase with the T in the studied range, which is different from the results without NaCl (Fig. 9b). Besides, as the T increases from 20 to 60 °C, the Sη, SG′ and SG′′ of solutions with NaCl increases by ∼35, ∼80 and ∼42%, respectively, while those without NaCl increases by ∼13, ∼15 and ∼9%, respectively, indicating that the hydrophobic interaction between HA-PAM and LAA was enhanced in the presence of NaCl.
 | ||
| Fig. 10 Effect of temperature on (a) dynamic viscosity and moduli of H/L solutions containing 0.02% LAA, (b) viscosity and modulus ratios of H/L to HA-PAM solutions, with NaCl concentration of 0.2%. | ||
4. Conclusions
The rheological behavior of binary mixed solution of a hydrophobically associating polyacrylamide (HA-PAM) and surfactants was investigated. Compared with anionic surfactant SDS, zwitterionic surfactant carbobetaine (C19H38N2O3) sulfobetaine (C17H37NSO3) and nonionic surfactant nonaethylene glycol monododecyl ether (C30H62O10), the nonionic surfactant laurel alkanolamide (LAA) exhibited stronger thickening ability when added to the HA-PAM solutions. The DPD simulation was used to interpret the synergistic mechanism. The obvious increase of solution viscosity was induced by the hydrophobic interaction between HA-PAM and LAA. The effects of electrolyte concentration and temperature on the interaction between HA-PAM and LAA were also investigated. The interaction strength of HA-PAM with LAA enhanced with the increase of Ca, CNaCl and temperature. Overall, the HA-PAM/LAA mixed solution exhibited stronger salt and temperature resistance than the HA-PAM solution. The HA-PAM/LAA systems are appropriate for use in EOR of oil field with high salinity.Acknowledgements
The funding of National Municipal Science and Technology Project (no. 2008ZX05011-002) and National Science Fund of China (no. 21173134 and 21473103) is gratefully acknowledged.References
- H. Zhang, M. Dong and S. Zhao, Energy Fuels, 2010, 24(3), 1829–1836 CrossRef CAS.
- D. A. Z. Wever, F. Picchioni and A. A. Broekhuis, Prog. Polym. Sci., 2011, 36(11), 1558–1628 CrossRef CAS PubMed.
- E. E. Kathmann, L. A. White and C. L. McCormick, Polymer, 1997, 38(4), 871–878 CrossRef CAS.
- A. Sabhapondit, A. Borthakur and I. Haque, Energy Fuels, 2003, 17(3), 683–688 CrossRef CAS.
- C. Zhong, L. Jiang and X. Peng, J. Polym. Sci., Polym. Chem. Ed., 2010, 48(5), 1241–1250 CrossRef CAS.
- C. L. McCormick, J. C. Middleton and D. F. Cummins, Macromolecules, 1992, 25(4), 1201–1206 CrossRef CAS.
- Q. Deng, H. Li, Y. Li, X. Cao, Y. Yang and X. Song, Aust. J. Chem., 2014, 67(10), 1396–1402 CrossRef CAS.
- P. A. Ioannis and S. Chronakis, Macromolecules, 2001, 34(14), 5005–5018 CrossRef.
- L. Y. C. Perry, F. C. Lim and S. B. Chen, J. Phys. Chem. B, 2003, 107(206), 6491–6496 Search PubMed.
- M. Y. Thomas, A. P. Seery, T. E. Hogen-Esch and E. J. Amis', Macromolecules, 1992, 25(18), 4784–4791 CrossRef.
- X. Xin, G. Xu, D. Wu, Y. Li and X. Cao, Colloids Surf., A, 2007, 305(1–3), 138–144 CrossRef CAS PubMed.
- I. Hoffmann, P. Heunemann, S. Prevost, R. Schweins, N. J. Wagner and M. Gradzielski, Langmuir, 2011, 27(8), 4386–4396 CrossRef CAS PubMed.
- K. Fukada, E. Suzuki and T. Seimiya, Langmuir, 1999, 15(12), 4217–4221 CrossRef CAS.
- P. Deo, N. Deo, P. Somasundaran, A. Moscatelli, S. Jockusch, N. J. Turro, K. Ananthapadmanabhan and M. F. Ottaviani, Langmuir, 2007, 23(11), 5906–5913 CrossRef CAS PubMed.
- A. Hersloef, L. O. Sundeloef and K. Edsman, J. Phys. Chem., 1992, 96(5), 2345–2348 CrossRef CAS.
- N. Plucktaveesak, A. J. Konop and R. H. Colby, J. Phys. Chem. B, 2003, 107(32), 8166–8171 CrossRef CAS.
- D. Zhuang, T. E. Hogen-Esch and Y. Zhang, J. Appl. Polym. Sci., 2004, 92(2), 1279–1285 CrossRef CAS.
- S. Zhang, P. Zhu, Y. Sun, Y. Yang, X. Cao, X. Song and Y. Li, RSC Adv., 2014, 4(79), 41831–41837 RSC.
- I. Iliopoulos, J. L. Halary and R. Audebert, J. Polym. Sci., Polym. Chem. Ed., 1988, 26(1), 275–284 CrossRef CAS.
- X. Cao, G. Xu, Y. Li and Z. Zhang, J. Phys. Chem. A, 2005, 109(45), 10418–10423 CrossRef CAS PubMed.
- Y. Zhao, L.-Y. You, Z.-Y. Lu and C.-C. Sun, Polymer, 2009, 50(22), 5333–5340 CrossRef CAS PubMed.
- K. S. Sharma, P. A. Hassan and A. K. Rakshit, Colloids Surf., A, 2007, 308(1–3), 100–110 CrossRef CAS PubMed.
- S. Ghosh, S. Mondal, S. Das and R. Biswas, Fluid Phase Equilib., 2012, 332, 1–6 CrossRef CAS PubMed.
- K. Y. Mya, A. Sirivat and A. M. Jamieson, J. Phys. Chem. B, 2003, 107(23), 5460–5466 CrossRef CAS.
- W. Seng, K. Tam and R. Jenkins, Colloids Surf., A, 1999, 154(3), 365–382 CrossRef CAS.
- M. Amiri and A. A. Dadkhah, Colloids Surf., A, 2006, 278(1), 252–255 CrossRef CAS PubMed.
- K. Tam, W. Seng, R. Jenkins and D. Bassett, J. Polym. Sci., Polym. Phys. Ed., 2000, 38(15), 2019–2032 CrossRef CAS.
- G. Zhao, C. C. Khin, S. B. Chen and B.-H. Chen, J. Phys. Chem. B, 2005, 109(29), 14198–14204 CrossRef CAS PubMed.
- X. Xie and T. E. Hogen-Esch, Macromolecules, 1996, 29(5), 1734–1745 CrossRef CAS.
- H. Bu, A.-L. Kjøniksen, K. D. Knudsen and B. Nyström, Colloids Surf., A, 2007, 293(1), 105–113 CrossRef CAS PubMed.
- W. Wang, Y. Liu and Y. Gu, Colloid Polym. Sci., 2003, 281(11), 1046–1054 CAS.
- J. Ma, B. Liang, P. Cui, H. Dai and R. Huang, Polymer, 2003, 44(4), 1281–1286 CrossRef CAS.
- R. Sadeghi and F. Jahani, J. Phys. Chem. B, 2012, 116(17), 5234–5241 CrossRef CAS PubMed.
- C. Zhong and P. Luo, J. Polym. Sci., Polym. Phys. Ed., 2007, 45(7), 826–839 CrossRef CAS.
- R. D. Wesley, C. A. Dreiss, T. Cosgrove, S. P. Armes, L. Thompson, F. L. Baines and N. C. Billingham, Langmuir, 2005, 21(11), 4856–4861 CrossRef CAS PubMed.
- K. Tam, W. Ng and R. Jenkins, Polymer, 2005, 46(12), 4052–4059 CrossRef CAS PubMed.
- V. D. Alves, F. Freitas, N. Costa, M. Carvalheira, R. Oliveira, M. P. Gonçalves and M. A. M. Reis, Carbohydr. Polym., 2010, 79(4), 981–988 CrossRef CAS PubMed.
- J. Ma, P. Cui, L. Zhao and R. Huang, Eur. Polym. J., 2002, 38(8), 1627–1633 CrossRef CAS.
- J. Desbrieres, Polymer, 2004, 45(10), 3285–3295 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14884c |
| This journal is © The Royal Society of Chemistry 2015 |