Characterization and prediction of blend properties and evaluation of engine performance and emission parameters of a CI engine operated with various biodiesel blends
Abstract
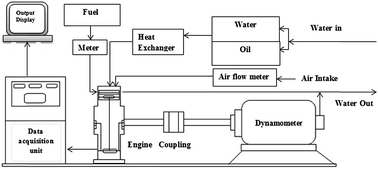
The present research is aimed to investigate the feasibility of using palm (PB), mustard (MB) and Calophyllum biodiesel (CB) as renewable and alternative fuels. Biodiesels were produced from the respective crude vegetable oils and physicochemical properties of the biodiesel–diesel blends were graphically compared for all possible biodiesel blends at every 10% composition interval. By applying the curve-fitting method, equations were developed for predicting important properties, which show very close fit to the experimental data. This will help future research such as the optimization of blending percentage, engine combustion and performance and emission analysis. As up to 20% blends of biodiesels showed similar properties to diesel fuel, the engine performance and emission of the 10% and 20% biodiesel–diesel blends were studied for all three feedstocks, as well as diesel fuel, to perform a comparative study. An average of 7–12% BSFC increment was observed for biodiesel blends compared to diesel fuel. The brake power was decreased on average of 4.1–7.7% while operating on the biodiesel blends. Nitric oxide (NO) emission increased 9–17% and hydrocarbon and carbon monoxide (CO) emission showed improved results for the biodiesel blends. An average of 23–43% lower HC and 45–68% lower CO emission resulted from the biodiesel blends compared to those from diesel fuel.

 Please wait while we load your content...
Please wait while we load your content...