Controlled synthesis of monodisperse silver nanoparticles supported layered double hydroxide catalyst†
Abstract
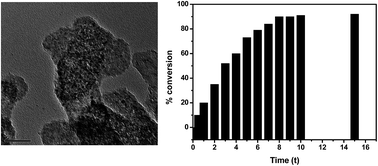
Silver nanoparticles supported layered double hydroxides (Ag NPs–LDH) were synthesized via a facile chemical reduction–deposition method. The successive anion exchange of the carbonate ions of ZnAl-(CO32−) LDH by chloride and citrate ions leads to citrate intercalated LDH (Cit-LDH). On hydration, the citrate anions in Cit-LDH can attract Ag+ ions by a superficial electrostatic force of attraction and reduce them to Ag nanoparticles. The preferential deposition of monodisperse Ag NPs was obtained on the outer surfaces of pillared LDH. The simultaneous formation of large colloidal Ag NPs of 25–30 nm in size in the aqueous phase was also found exterior to the LDH gallery. The nanoparticles deposited on the LDH surfaces are highly dispersed with a narrow size distribution of ∼2.5 nm and are stabilized on the LDH support. The Ag NPs deposited LDH nanosheets were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM) and Fourier transform infrared (FTIR) spectroscopy. The resulting monodisperse Ag NPs deposited LDH materials offer heterogeneous interactions at the interface and exhibit high catalytic activity on alcohol oxidation.

 Please wait while we load your content...
Please wait while we load your content...