Environmentally benign, recyclable nano hollandite and metal intercalated nano hollandites for hydrogen sulfide removal
Abstract
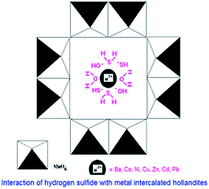
Hollandite, a 2 × 2 tunnel structure, octahedral molecular sieve (OMS-2) with Ba2+ as counter cation was prepared and characterized by PXRD, IR, SEM, EDX, HRTEM, XPS and TG-DTA techniques. HRTEM of the hollandite showed the particles to be nanorods (<20 nm) and the SAED confirmed its crystalline nature. Seven metal intercalated hollandites were prepared by ion exchange process. Metal intercalated hollandites were analyzed by ICP-OES. The metal ion intercalation was in the range of 1.09 to 2.99% by weight. Order of increasing concentrations of divalent metal ions on intercalation is: Co < Zn < Ni < Cu < Cd < Pb. The observed order is an indication of the exchanging ability of the divalent ion with Ba2+ ion of the hollandite in aqueous medium. Thermal analysis of H2S passed hollandite showed the hollandite to be a superior scrubber over birnessite. Maximum scrubbing of H2S (27%) was observed for copper intercalated hollandite and a minimum (22%) for zinc intercalated hollandite. The involvement of copper in scrubbing is important because of the Cu2+⋯SHn, a typical soft–soft interaction. Overall H2S scrubbing ability followed the order: birnessite (18%) < hollandite (20%) < metal ion intercalated hollandite (27%) and the scrubbing efficiency is remarkable for a dry scrubber under laboratory conditions. Hydrogen sulfide scrubbed hollandite showed relatively poor recyclability compared to intercalated material. Intercalated metal ion increases structural integrity, stabilizes the active metal against reduction and increases the scrubbing ability. The scrubber is recyclable by a simple heating process. The scrubber is benign to nature as it's a synthetic analogue of naturally occurring marine nodule type of material.

 Please wait while we load your content...
Please wait while we load your content...