Shape-dependent performance of TiO2 nanocrystals as adsorbents for methyl orange removal†
Min Zhuang,
Yifan Zheng,
Zongjian Liu*,
Wanzhen Huang and
Xianchao Hu
Institute of Industrial Catalysis, College of Chemical Engineering, Zhejiang University of Technology, Hangzhou, 310014, P.R. China. E-mail: zjliu@zjut.edu.cn
First published on 19th January 2015
Abstract
Two types of TiO2 nanocrystals, namely {001} plane dominated nanoplates and {101} faceted nanobipyramids prepared by an oleylamine-containing alcohothermal method in the presence or absence of HF, have been used as adsorbents for removal of methyl orange (MO) from water. Transmission electron microscopy investigation, along with X-ray diffraction analysis, reveals that the average width of the nanoplates is about 13 nm with a thickness of ∼4 nm, and the nanobipyramids posses a length in the c-axis of about 18 nm and a central width of about 11 nm. Although the surface area of TiO2 nanoplates and nanobipyramids are, respectively, about 125.5 and 27.6 m2 g−1, the latter exhibits a much better adsorption performance than the former. In the case of 200 ml of MO aqueous solution with an initial concentration of 50 μmol L−1 (pH = 3), the removal efficiency of MO on TiO2 nanobipyramids (40 mg) reaches about 95% within a contact time of 10 min whereas only ∼65% of MO is removed by TiO2 nanoplates after 60 min treatment. Further investigations indicate that this shape-dependent adsorption performance is related to the fact that the existence of fluorine on the surface of the nanoplates is unfavorable for the adsorption of MO and the interaction between MO (through its sulfonate group) and nanoplates is weaker than that between MO and nanobipyramids. The adsorption of oleylamine on the surfaces of nanobipyramids and nanoplates that may affect the interaction between the MO and TiO2 surface has also been discussed based on the experimental results.
Introduction
Titanium dioxide (TiO2), an earth-abundant inorganic material of low toxicity and high chemical stability, possesses a wide range of applications, from industrial use in the field of cosmetics or paints to potential application as photocatalysts for environmental remediation.1–10 TiO2 has three naturally-existing polymorphs, namely anatase, rutile, and brookite, among which anatase has been found to be the most photocatalytically active. Since the activity of a photocatalyst can be influenced by its crystal facets which are directly exposed to the reaction media,11,12 the tailoring of anatase TiO2 nanocrystals with desired facets is of great importance for improvement of their activity. Thermodynamically, a bipyramid-shaped anatase with exposed {101} facets is the most stable form because the surface energy follows the order of {101} < {010} < {001}.13,14 Technically, therefore, the synthesis of anatase TiO2 nanocrystals with facets of high surface energy, e.g., {001} facets, has been a huge challenge until Yang et al. demonstrated that the introduction of HF during the hydrothermal synthesis of TiO2 can stabilize the {001} facets and thus result in the formation of anatase TiO2 single crystals with 47% exposed {001} facets.15 Since the {001} facets were reported to possess higher reactivity compared to the {101} facets due to having a higher concentration of low coordination Ti centers,16 the breakthrough work reported by Yang et al. has created a surge of interest in the synthesis and investigation of anatase TiO2 crystals with different exposed facets.17–22To date, most of the already-reported studies focus on the photo-induced reactivity, e.g. water splitting17–19 and degradation of organic pollutants,20–22 of anatase TiO2 crystals with different exposed facets. To the best of the authors' knowledge, there is no work focusing on the adsorption performance (e.g. as adsorbents for organic pollutant removal) of those different faceted anatase crystals. From a practical point of view, surface adsorption is an important industrial way for the removal of organic pollutants, and recently nanosized TiO2 has been found to be an effective adsorbent for reactive dyes.23–25 Even in the case of photodegradation of organic pollutants, the adsorption performance of TiO2 is also an important factor for determining the degradation efficiency.26,27 Therefore, an investigation of the adsorption performance of different faceted anatase crystals is of fundamental importance for their applications as both adsorbents and photocatalysts.
In this work, {001} plane dominated nanoplates and {101} faceted nanobipyramids were used to investigate the shape-dependent adsorption performance. These nanoplates or nanobipyramids were synthesised by an oleylamine-containing alcohothermal method in the presence or absence of HF. Methyl orange (MO), a model compound for water-soluble azo dyes widely used in chemical, textile and paper industries, was selected as an adsorbate for evaluating the adsorption ability of two types of TiO2 nanocrystals.
Experimental
Synthesis and NaOH treatment of TiO2 nanocrystals
All chemicals were used as received without further purification. The synthesis of TiO2 nanoplates and nanobipyramids was accomplished using a solvothermal method in the presence of oleylamine. Typically, 44 ml of oleylamine, 20 ml of ethanol and 6 ml of tetrabutyl titanate were mixed. The obtained mixture was stirred in a Teflon cup for about 10 min before being transferred into a 100 ml Teflon-lined stainless steel autoclave containing 2 ml of water (for preparation of TiO2 nanobipyramids) or 2 ml of 8% HF aqueous solution (for preparation of TiO2 nanoplates). The autoclave was then kept at 180 °C (for TiO2 nanobipyramids) or 200 °C (for TiO2 nanoplates) for 14 h in an electric oven. After completion of the reaction, the autoclave was taken out and cooled to room temperature naturally. The product was collected by washing the precipitate thoroughly with absolute ethanol and deionized water for at least five times to remove the residual contamination, and then dried.The removal of fluorine on the surface of TiO2 nanoplates was achieved by a NaOH treatment. The experiment was conducted at room temperature. Typically, 0.5 g of as-prepared TiO2 nanocrystals was added into 30 ml of 0.1 mol L−1 NaOH aqueous solution. The mixture was magnetically stirred for 30 min and then centrifuged at a speed of 4000 rpm. This process is repeated for several times. After the NaOH treatment, the obtained TiO2 was washed thoroughly with absolute ethanol and deionized water, and then dried.
Characterization of TiO2 nanocrystals
The shape and microstructure of TiO2 nanocrystals were determined with a Tecnai G2 F30 S-Twin transmission electron microscopy (TEM) operating at 300 kV. The phase composition of the samples was analyzed by X-ray diffraction (XRD) on a PANanalytical X'Pert PRO X-ray diffractometer using Cu Kα X-ray source. Brunauner–Emmett–Teller (BET) surface area was measured using low-temperature nitrogen adsorption on a Micromeritics ASAP 2010 sorptometers. X-ray Photoelectron Spectra (XPS) were obtained using a Kratos AXIS Ultra DLD spectrometer with an Al Ka source (at 1486.6 eV). Infrared Fourier transform spectra (FT-IR) were recorded on a Thermo Scientific-Nicolet 6700 spectrophotometer.Adsorption test
The adsorption experiments were carried out under dark conditions at a temperature of 25 °C. The initial pH value of MO solution is fixed at 3. Typically, 40 mg of TiO2 adsorbent was added to 200 ml of MO solution (the initial concentration is 50 μmol L−1 or 100 μmol L−1), and then the mixture was magnetically stirred. At given time interval, about 5 ml of suspension was taken out from the stirred mixture and then centrifuged to remove the adsorbent. The resulting solution was measured by a UV-vis 7600 spectrophotometer (Shanghai Jinghua Instruments), and the MO concentration was calculated from the calibration curve.To obtain adsorption isotherms (at 25 °C), 200 ml of MO solution with different concentrations (ranging from 10 to 100 μmol L−1) were mixed with 4 mg of TiO2 adsorbents and then stirred for 120 min. The equilibrium adsorption capacity (qe) was determined according to the following formula
Results and discussion
Characterization of TiO2 nanocrystals
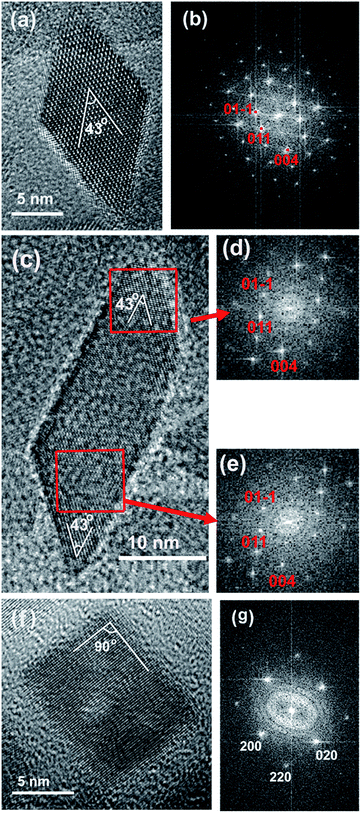
Fig. 1 presents the TEM images of TiO2 nanocrystals prepared by a solvothermal method in the present of oleylamine and small amount of water. In the absence of HF, most of as-grown nanocrystals exhibit a shape of nanobipyramids (indicated by red arrows in Fig. 1a). The size of these nanobipyramids is not uniform with a length in c-axis ranging from 15–25 nm. The high resolution TEM (HRTEM) image of a TiO2 nanobipyramid clearly discloses its microstructural feature (see Fig. 2a). The corresponding Fast Fourier Transformation (FFT) image (see Fig. 2b) indicates that the electron beam penetrates the nanobipyramid along its [100] zone axis and the observed two lattice fringes with an angle of about 43° correspond, respectively, to the (011) and (01−1) planes. Besides the {101} faceted nanobipyramids, a few rod-like nanocrystals can also be found in the sample (indicated by blue arrows in Fig. 1a). It is interesting to note that part of rod-like nanocrystals also possess a feature similar to that of the nanobipyramids. As shown in Fig. 2c, the elongated nanocrystal is not nanobipyramids. However, the FFT images of its two ends (see Fig. 2d and e) are the same as that of the nanobipyramid (see Fig. 2b), indicating that this rod-like nanocrystal also has a preferential exposed {101} facets. The addition of HF in the growth of TiO2 nanocrystals can change their morphology from nanobipyramids into well-shaped nanoplates (see Fig. 1b). The average width of these nanoplates is estimated to be about 13 nm. The HRTEM image of a TiO2 nanoplate is presented in Fig. 2f. Its FFT image exhibits a typical diffraction pattern of anatase along [001] zone axis (see Fig. 2g), indicating that the exposed face (top or bottom) of the nanoplates is the {001} planes and the observed two lattice fringes with an angle of about 90° correspond, respectively, to the (100) and (010) planes. The formation of {001} faceted TiO2 nanoplates in the presence of HF is generally believed to be a result from the surface fluorine, which may lower the surface energy of {001} facets and thus preserve the {001} faceted surfaces in the growth of anatase TiO2 crystals.15 | ||
| Fig. 1 TEM images of the TiO2 nanocrystals with different shapes: (a) nanobipyramids and (b) nanoplates. Scale bar is 100 nm. | ||
The XRD patterns of these TiO2 nanocrystals are shown in Fig. 3. The peaks at 25.3°, 37.8°, 48.0°, 53.9°, 55.1°, 62.7°, 68.8°, 70.3°, and 75.1° of all samples can be indexed to anatase phase (JCPDS 21-1272) and correspond to the diffractions of (101), (004), (200), (105), (211), (204), (116), (220), and (215) planes, respectively. A calculation of the crystal sizes along different directions based on the Scherrer's equation is shown in Table 1. For the TiO2 nanobipyramids, the size along the 〈001〉 and 〈100〉 are very close (about 11 nm), but the size along the 〈001〉 direction (namely the length in c-axis) has a larger value of about 18 nm. These calculated values are roughly consistent with the TEM observations. For the TiO2 nanoplates, however, the size along the 〈001〉 direction is much smaller than that along 〈101〉 or 〈100〉 direction, confirming that they possess a plate-like shape. The thickness of these nanoplates estimated from the size along the 〈001〉 direction is about 4 nm. The calculated size along 〈101〉 or 〈100〉 direction, namely 12 nm or 13 nm is good agreement with the value observed by TEM (see Fig. 1b). The observation that TiO2 nanoplates have a smaller crystal size than the nanobipyramids is also consistent with the order of their BET surface areas. The BET surface area of the as-prepared nanoplates and nanobipyramids determined using low-temperature nitrogen adsorption are, respectively, about 125.5 and 27.6 m2 g−1.
| Sample | 〈001〉 | 〈101〉 | 〈100〉 |
|---|---|---|---|
| Nanoplates | ∼4 nm | ∼13 nm | ∼12 nm |
| Nanobipyramids | ∼18 nm | ∼11 nm | ∼11 nm |
Adsorption of MO on TiO2 nanobipyramids and nanoplates
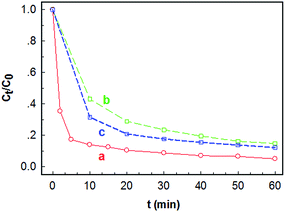
Fig. 4 presents the variation of Ct/C0 (Ct is the concentration of MO at contact time t) with contact time t on different adsorbents. Although the TiO2 nanoplate sample has a much higher BET surface area than the nanobipyramid sample, its adsorption ability for MO is unexpectedly low. As shown in curve a in Fig. 4, the removal efficiency of MO on the TiO2 nanoplates is about 65% within a contact time of ∼60 min when C0 is 50 μmol L−1 (namely about 16.4 mg L−1). In contrast, the TiO2 nanobipyramid sample shows much better performance for removal of MO despite its surface area being only 27.6 m2 g−1. In the case where C0 is 50 μmol L−1, for example, most MO molecules are removed by TiO2 nanobipyramids with a removal efficiency of about 95% within a contact time of only 10 min (see curve b in Fig. 4). Even when the initial concentration of MO is doubled, the removal efficiency may also reach ∼95% within a contact time of 50 min (see curve c in Fig. 4). These results imply that the adsorption performances of MO on TiO2 nanobipyramids and nanoplates are quite different. Moreover, this shape-dependent performance is difficult to be explained in terms of the values of the BET surface area.It is reported that the surface fluorine on the {001} planes can reduce the photocatalytic activity of {001} facted TiO2 nanocrystals.28,29 Since the existence of fluorine on the TiO2 nanoplates is a well-known fact during the synthesis of {001} facted TiO2 nanocrystals, to investigate the influence of surface fluorine on the adsorption performance of TiO2 nanoplates, we have tried to remove the fluorine from the TiO2 nanoplates via calcination or NaOH treatment.30,31 Although Lv et al. have reported that anchored F atoms can stabilize the anatase structure at high temperature,32 we find that the shape of TiO2 nanoplates is unstable during annealing. After calcination of the nanoplate sample at a temperature of 500 °C for 4 h, the plate-like shape disappears (see the Fig. 1s in ESI†). Therefore, the NaOH treatment was selected for the removal of fluorine. The XPS measurement (see Fig. 5) indicates that after treated in a 0.1 mol L−1 NaOH solution for 6 times, most of fluorine on the surface of TiO2 nanoplates has been removed. Fig. 6 shows the time-dependant Ct/C0 for the NaOH-treated TiO2 nanoplate adsorbents. For comparison, the adsorption performance of TiO2 nanobipyramids after a similar NaOH treatment (3 times) is also shown. A comparison between the results shown in Fig. 4 and 6 reveals that, for the TiO2 nanobipyramids, the NaOH treatment almost has no impact on their adsorption performance. However, such a treatment may improve the adsorption performance of TiO2 nanoplates. The removal efficiency within the contact time of 60 min is increased from ∼65% to ∼85% after NaOH treatment for 6 times, indicating that the existence of surface fluorine on TiO2 nanoplates is unfavorable for the adsorption of MO. However, even after the removal of fluorine from the surface of TiO2 nanoplates, they still possess a weaker ability to adsorb MO in comparison with TiO2 nanobipyramids.
 | ||
| Fig. 5 XPS survey spectra of the TiO2 nanoplates treated in a NaOH aqueous solution for different times: (a) untreated, (b) 3 times, and (c) 6 times. | ||
To get an insight into the adsorption behaviour of MO on the surface of TiO2 nanocrystals and thus a better understanding of the above experimental results, the adsorption isotherms on both nanoplates and NaOH-treated nanobipyramids (treated for 3 times) were measured and then analyzed in terms of two well-known models, namely, Langmuir and Freundlich adsorption isotherms. The Langmuir isotherm assumes that adsorption occurs on a surface containing uniform, independent adsorption sites and once the adsorption sites are occupied by adsorbates, no further adsorption can occur on these sites.33 This monolayer adsorption can be described by
 | (1) |
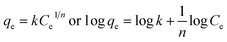 | (2) |
To explain the difference in adsorption performance between TiO2 nanoplates and nanobipyramids, the understanding of the interaction between MO and TiO2 surface is essential. Murcia et al. have reported that the adsorption of MO on TiO2 surface occurs via interaction of the azo group (R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–R) with surface Ti4+.26 This indicates that, when a MO molecule is adsorbed on the crystal plane of TiO2 nanocrystals, its long axis will be parallel to, rather than perpendicular to, the crystal plane (see Fig. 8a). In order to understand the interaction between MO and TiO2 nanoplates or nanobipyramids in our case, their IR spectra after adsorption of MO are depicted in Fig. 9. For comparison, the IR spectrum of MO is also shown. Two features can be found in these IR spectra. (i) Two bands located at ∼1608 and 1518 cm−1, one band at 1366 cm−1, and three bands in the range between 1000 and 1200 cm−1 can be found in all samples. These bands can be, respectively, attributed to the –CC vibrations in the aromatic rings, –N
N–R) with surface Ti4+.26 This indicates that, when a MO molecule is adsorbed on the crystal plane of TiO2 nanocrystals, its long axis will be parallel to, rather than perpendicular to, the crystal plane (see Fig. 8a). In order to understand the interaction between MO and TiO2 nanoplates or nanobipyramids in our case, their IR spectra after adsorption of MO are depicted in Fig. 9. For comparison, the IR spectrum of MO is also shown. Two features can be found in these IR spectra. (i) Two bands located at ∼1608 and 1518 cm−1, one band at 1366 cm−1, and three bands in the range between 1000 and 1200 cm−1 can be found in all samples. These bands can be, respectively, attributed to the –CC vibrations in the aromatic rings, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration (the azo group), and the vibrations from sulfonate group of MO molecules. The intensity of these bands is lower than that of pure MO, indicating that MO molecules are adsorbed on the surface of TiO2 nanocrystals. (ii) For pure MO, the band ascribed to S
N vibration (the azo group), and the vibrations from sulfonate group of MO molecules. The intensity of these bands is lower than that of pure MO, indicating that MO molecules are adsorbed on the surface of TiO2 nanocrystals. (ii) For pure MO, the band ascribed to S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode of sulfonate group is located at 1196 cm−1. After adsorption on TiO2 nanoplates or nanobipyramids, this band shifts to 1184 or 1161 cm−1. This observation hints that the interaction of the MO molecule with TiO2 surface is through its sulfonate (SO3−) group. Moreover, the bigger shift observed in the nanobipyramid sample suggests a stronger interaction between the MO molecules and the surface of TiO2 nanobipyramids in comparison with TiO2 nanoplates. This observation is consistent with the result derived from the adsorpton isotherm based on the Langmuir model.
O stretching mode of sulfonate group is located at 1196 cm−1. After adsorption on TiO2 nanoplates or nanobipyramids, this band shifts to 1184 or 1161 cm−1. This observation hints that the interaction of the MO molecule with TiO2 surface is through its sulfonate (SO3−) group. Moreover, the bigger shift observed in the nanobipyramid sample suggests a stronger interaction between the MO molecules and the surface of TiO2 nanobipyramids in comparison with TiO2 nanoplates. This observation is consistent with the result derived from the adsorpton isotherm based on the Langmuir model.
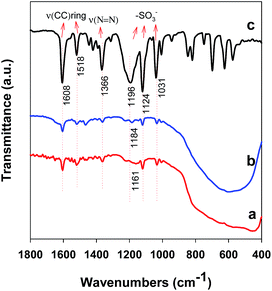 | ||
| Fig. 9 IR spectra of the samples after adsorption of MO: (a) nanobipyramids and (b) nanoplates. For comparison, the IR spectrum of MO (c) is also shown. | ||
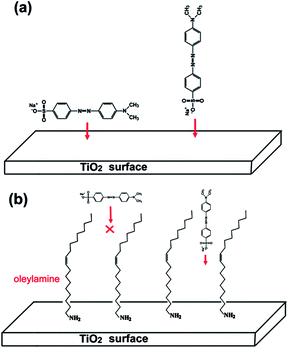
The interaction of the MO molecule with TiO2 surface through its sulfonate group suggests that the MO molecule is adsorbed with its long axis perpendicular to the crystal plane (see Fig. 8a), which is different from the case reported by Murcia et al.26 The difference might be related to the fact that in our case oleylamine molecules exist on the surface of TiO2 nanocrystals even after thorough washing. As can be seen from Fig. 10, two bands at 2855 and 2923 cm−1, which can be, respectively, assigned to the –CH2 symmetric and asymmetric stretching vibrations, appear in the IR spectra of both as-prepared nanobipyramids and nanoplates. This indicates the adsorption of the oleyl group on the TiO2 surface. In addition, the band at 3320 cm−1 belonged to the N–H stretching mode cannot be observed probably due to the interaction between –NH2 group and TiO2 surface. It is reported that the oleylamine molecule has a length of more than 2 nm and a “width” of 0.7 nm.35 The MO molecule is also not very small, and there are two benzene rings, three C–N bonds, one N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, N–C, S–C, S–O bond along its long axis (see Fig. 8a). The length of a MO molecule is thus estimated is more than 1 nm in terms of bond length. When a MO molecule approaches the surface of TiO2, its long axis tends to be perpendicular to, rather than parallel to, the crystal plane due to the steric hindrance of the adsorbed oleylamines (see Fig. 8b). This perpendicular adsorption mode is expected to be helpful for the enhancement of the adsorption amount of TiO2 because only one end of a MO molecule (rather than the whole molecule) need occupy the surface.
N, N–C, S–C, S–O bond along its long axis (see Fig. 8a). The length of a MO molecule is thus estimated is more than 1 nm in terms of bond length. When a MO molecule approaches the surface of TiO2, its long axis tends to be perpendicular to, rather than parallel to, the crystal plane due to the steric hindrance of the adsorbed oleylamines (see Fig. 8b). This perpendicular adsorption mode is expected to be helpful for the enhancement of the adsorption amount of TiO2 because only one end of a MO molecule (rather than the whole molecule) need occupy the surface.
 | ||
| Fig. 10 IR spectra of as-prepared nanobipyramids (a) and nanoplates (b). For comparison, the IR spectrum of oleylamine (c) is also shown. | ||
The stronger interaction between the MO molecules and the surface of TiO2 nanobipyramids observed in IR spectra should be a main factor responsible for the result that TiO2 nanobipyramids have a better adsorption performance than TiO2 nanoplates. This stronger interaction, however, is difficult to be understood in terms of the structure of clean TiO2 {101} and {001} surfaces. On the clean {001} planes, all the Ti atoms on the top layer are undercoordinated Ti atoms, namely 5-fold coordinated (Ti5c) (see Fig. 11a), whereas only half of Ti atoms are undercoordinated Ti5c on the {101} planes (see Fig. 11b). Since the undercoordinated Ti5c may act as the acid center for interaction with the sulfonate group of MO (serving as a strong base), a relatively strong interaction between MO molecules and {001} facet is expected due to its large percentage of undercoordinated Ti5c and thus a better adsorption performance should be observed on the {001} facet dominated nanoplates. However, this inference is contradictory to our experimental observation. In the case of photocatalysis, Gordon et al. have also reported a similar result that the {101} facets of anatase are more active than the {001} facets for photo-reforming of methanol.36 We speculate that the contradiction between the deduction and the experimental result might be related to the fact that the existence of clean TiO2 surface seems to be impossible due to the exposure to the solvent such as water or organics during the synthesis or post-treatment. The undercoordinated Ti5c will interact with these solvent molecules to form “six-fold” coordinated structure before serving as adsorbent or photocatalyst. In some cases, these adsorbed molecules or functional groups, which may result in a change in the spatial or electronic structure of the surface, can hinder the adsorbates or reactants approaching the TiO2 surface. Since only half of Ti atoms are undercoordinated Ti5c on the clear {101} surface, the hindering effect caused by these adsorbed molecules or functional groups should be weaker than that on the {001} surface. As a result, {101} faceted nanobipyramids may exhibit a better performance. In our case, the {001} facets are exposed to HF, oleylamine and ethanol molecules during the synthesis, and Ti5c will preferentially interacts with F−. Since F− is a strong base, the relatively high electron density provided by F− will reduce the positive charge of the surface and thus is unfavorable for the interaction with MO through its negatively charged sulfonate group. The NaOH treatment leads to the replacement of surface fluorine with OH−, forming the surface hydroxyl group –Ti–OH. Although OH− is a stronger base than F− and thus a more obvious reduction in the surface positive charge is expected, the result that the remove of surface fluorine via NaOH treatment enhances the adsorption performance of TiO2 nanoplates might be related to the possibility of the interaction of MO sulfonate group with the surface hydroxyl group, e.g. the formation of hydrogen bonds as proposed by Asuha et al.24
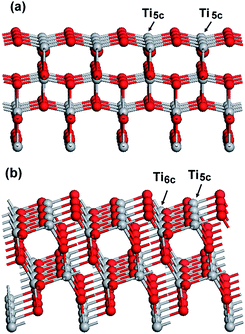 | ||
| Fig. 11 Clear surface structure of different planes: (a) {001} and (b) {101}. Ti5c and Ti6c denote 5-fold and 6-fold coordinated Ti atoms, respectively. | ||
Conclusions
{001} plane dominated TiO2 nanoplates and {101} faceted nanobipyramids prepared by an oleylamine-containing alcohothermal method have been employed to investigate their adsorption performance for MO removal. Although the TiO2 nanoplates possess a smaller crystal size and thus a larger specific surface area in comparison with the nanobipyramids, the removal efficiency of MO on the TiO2 nanoplates is much lower than that on the nanobipyramids. This shape-dependent adsorption performance should be related to the fact that the existence of fluorine on the surface of the nanoplates is unfavorable for the adsorption of MO and the interaction between MO (through its sulfonate group) and nanoplates is weaker than that between MO and nanobipyramids. The oleylamine molecules, which are also found to be adsorbed on the surface of both TiO2 nanobipyramids and nanoplates, play an important role in the interaction of MO with TiO2 surface by affecting the MO adsorption mode.Notes and references
- C. Y. Su, H. Z. Tang, K. Chu and C. K. Lin, Ceram. Int., 2014, 40, 6903 CrossRef CAS PubMed.
- G. Subbiah, M. Premanathan, S. J. Kim, K. Krishnamoorthy and K. Jeyasubramanian, Mater. Express, 2014, 4, 393 CrossRef CAS PubMed.
- J. Y. Ma, D. Xiang, Z. Q. Li, Q. Li, X. K. Wang and L. Y. Yin, CrystEngComm, 2013, 15, 6800 RSC.
- Y. Xu, E. M. Lotfabad, H. L. Wang, B. Farbod, Z. W. Xu, A. Kohandehghan and D. Mitlin, Chem. Commun., 2013, 49, 8973 RSC.
- X. W. Huang and Z. J. Liu, Nano-Micro Lett., 2013, 5, 93 CrossRef CAS.
- H. Chen, J. Y. Wang, Y. Z. Zhao and X. J. Han, RSC Adv., 2014, 4, 47031 RSC.
- L. Zeng, W. L. Song and C. S. Xie, RSC Adv., 2014, 4, 36708 RSC.
- Y. Yang, G. Z. Wang, Q. Deng, S. H. Kang, D. H. L. Ng and H. J. Zhao, CrystEngComm, 2014, 16, 3091 RSC.
- Q. Sun, X. M. Sun, Y. Li, L. Y. Yu and L. F. Dong, Sci. Adv. Mater., 2013, 5, 1221 CrossRef CAS PubMed.
- X. W. Huang and Z. J. Liu, Surf. Coat. Technol., 2013, 232, 224 CrossRef CAS PubMed.
- P. A. M. Hotsenpiller, J. D. Bolt, W. E. Farneth, J. B. Lowekamp and G. S. Rohrer, J. Phys. Chem. B, 1998, 102, 3216 CrossRef CAS.
- T. Ohno, K. Sarukawa and M. Matsumura, New J. Chem., 2002, 26, 1167 RSC.
- M. Lazzeri, A. Vittadini and A. Selloni, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 155409 CrossRef.
- U. Diebold, Surf. Sci. Rep., 2003, 48, 53 CrossRef CAS.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. K. Lu, Nature, 2008, 453, 638 CrossRef CAS PubMed.
- A. S. Barnard and L. A. Curtiss, Nano Lett., 2005, 5, 1261 CrossRef CAS.
- X. H. Yang, L. Zhen, G. Liu, J. Xing, C. H. Sun, H. G. Yang and C. Z. Li, CrystEngComm, 2011, 13, 1378 RSC.
- J. G. Yu, L. F. Qi and M. Jaroniec, J. Phys. Chem. C, 2010, 114, 13118 CAS.
- X. X. Yao, T. Y. Liu, X. H. Liu and L. D. Lu, Chem. Eng. J., 2014, 255, 28 CrossRef CAS PubMed.
- W. Wang, C. H. Lu, Y. R. Ni and Z. Z. Xu, CrystEngComm, 2013, 15, 2537 RSC.
- R. Menzel, A. Duerrbeck, E. Liberti, H. C. Yau, D. McComb and M. S. P. Shaffer, Chem. Mater., 2013, 25, 2137 CrossRef CAS.
- S. Y. Zhu, S. J. Liang, Q. Gu, L. Y. Xie, J. X. Wang, Z. X. Ding and P. Liu, Appl. Catal., B, 2012, 119–120, 146 CrossRef CAS PubMed.
- V. Belessi, G. Romanos, N. Boukos, D. Lambropoulou and C. Trapalis, J. Hazard. Mater., 2009, 170, 836 CrossRef CAS PubMed.
- S. Asuha, X. G. Zhou and S. Zhao, J. Hazard. Mater., 2010, 181, 204 CrossRef CAS PubMed.
- T. B. Nguyen, M. J. Hwang and K. S. Ryu, Appl. Surf. Sci., 2012, 258, 7299 CrossRef CAS PubMed.
- J. J. Murcia, M. C. Hidalgo, J. A. Navío, J. Arana and J. M. Dona-Rodríguez, Appl. Catal., B, 2014, 150–151, 107 CrossRef CAS PubMed.
- F. T. Chen, Z. Liu, Y. Liu, P. F. Fang and Y. Q. Dai, Chem. Eng. J., 2013, 221, 283 CrossRef CAS PubMed.
- C. Sun, A. Selloni, A. Du and S. C. Smith, J. Phys. Chem. C, 2011, 115, 17092 CAS.
- G. Liu, C. Sun, H. G. Yang, S. C. Smith, L. Wang, G. Q. Lu and H. M. Cheng, Chem. Commun., 2010, 46, 755 RSC.
- X. Han, Q. Kuang, M. Jin, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2009, 131, 3152 CrossRef CAS PubMed.
- M. Minella, M. G. Faga, V. Maurino, C. Minero, E. Pelizzetti, S. Coluccia and G. Martra, Langmuir, 2010, 26, 2521 CrossRef CAS PubMed.
- Y. Y. Lv, L. S. Yu, H. Y. Huang, H. L. Liu and Y. Y. Feng, Appl. Surf. Sci., 2009, 255, 9548 CrossRef CAS PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1916, 38, 2221 CrossRef CAS.
- T. W. Weber and R. K. Chakkravorti, AIChE J., 1974, 20, 228 CrossRef CAS.
- J. Borges, J. A. Ribeiro, E. M. Pereira, C. A. Carreira, C. M. Pereira and F. Silva, J. Colloid Interface Sci., 2011, 358, 626 CrossRef CAS PubMed.
- T. R. Gordon, M. Cargnello, T. Paik, F. Mangolini, R. T. Weber, P. Fornasiero and C. B. Murray, J. Am. Chem. Soc., 2012, 134, 6751 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14636k |
| This journal is © The Royal Society of Chemistry 2015 |





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)