Vilsmeier cyclization of α-acetyl-α-aroyl ketene-N,S-acetals: direct and efficient synthesis of halogenated pyridin-2(1H)-ones†
Haifeng Yu*a,
Yongmei Zhanga,
Tiechun Lia,
Peiqiu Liao*b,
Quanping Diaoa,
Guang Xina,
Qingling Menga and
Dongyan Houa
aSchool of Chemistry and Life Science, Anshan Normal University, Anshan, Liaoning 114007, China. E-mail: yuhf68105@sina.com; Fax: +86-412-2960065
bDepartment of Chemistry, Northeast Normal University, Changchun, Jilin 130024, China
First published on 12th January 2015
Abstract
An efficient synthetic route to 3-aroyl-5-formyl-4-halo pyridin-2(1H)-ones has been developed via Vilsmeier cyclization of 2-(ethylthio(arylamino)methylene)-1-alkylbutane-1,3-dione. The synthetic protocol has demonstrated the first example of Vilsmeier cyclization of α-acetyl-α-aroylketene-N,S-acetals, providing a novel route to 4-bromo/chloro pyridin-2(1H)-ones.
Pyridin-2(1H)-ones are very important heterocycles in medicinal chemistry and pharmacology due to their diverse pharmacological and biological activities.1,2 Halogenated pyridin-2(1H)-ones are an important subset of pyridin-2(1H)-ones and have been utilized as versatile synthetic intermediates for further elaboration of the pyridine-2(1H)-one core3 and a wide variety of potent bioactive heterocycles.4 Although the halogenation of pyridin-2(1H)-ones occurs very easily, a mixture of mono- and dihalogenated products is often obtained.5 Consequently, the direct preparation of halogenated pyridine-2(1H)-ones from acyclic substrates is a worthy research challenge. Marcoux and co-workers investigated the annulation reaction of esters bearing active methane with vinamidinium hexafluorophosphate salts, and 5-chloro pyridine-2(1H)-ones were successfully obtained.6 Dechoux et al. efficiently prepared 3-bromo pyridine-2(1H)-ones by bromo-cyclization of the ensuing δ-dienaminoester.7 Zhu and co-workers reported the synthesis of 3-fluoro-4-aryl-pyridine-2(1H)-ones from Michael-adduct of fluoronitroacetate and α,β-unsaturated ketones.8 Recently, Dong, et al. researched in detail Vilsmeier cyclization of 3-oxo-N-phenylbutanamide derivatives9,10 such as 1-acetyl-N-arylcycl-opropanecarboxamides,10a α-acetyl-α-carbamoylketene dithioacetals,10b 2-arylamino-3-acetyl-5,6-dihydro-4H-pyrans,10c β-oxo amides10d and 2-((dimethylamino)methylene)-3-oxo-N-arylbutanamides,10e and a variety of halogenated pyridine-2(1H)-ones were efficiently synthesized in good yields (Scheme 1A). Compared with other work,6–8 this Vilsmeier cyclization efficiently afforded halogenated pyridine-2(1H)-ones bearing formyl groups, which may render them extremely versatile as synthons in further synthetic transformations, and was characterized by good generality, simple execution, readily available substrates, and mild conditions. As a result, these advantages make the Vilsmeier cyclization of acyclic substrates to yield halogenated pyridine-2(1H)-ones very attractive.
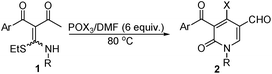
Readily available ketene-N,S-acetals have emerged as versatile intermediates in the synthesis of heterocyclic compounds.11 Over the past decades, great attention was focused on the investigation of α-aroyl/cyano ketene-N,S-acetals, because their α-position can act as nucleophile and react with electrophilic species resulting in their further functionalization and subsequent transformations to a variety of heterocycles.12 To date, the investigation on the synthesis and application of α-acetyl-α-aroyl ketene-N,S-acetals are not reported. As part of our contributions to the chemistry of ketene-S,S-acetals,13–15 we recently investigated the synthesis of α-acetyl-α-aroyl ketene-N,S-acetals and examined their reactions with Vilsmeier reagents. We found that 2-(ethylthio(arylamino)methylene)-1-alkylbutane-1,3-dione 1 could stereoselectively be synthesized in excellent yields, followed by its efficient Vilsmeier cyclization to afford 3-arolyl-5-formyl-4-halo pyridin-2(1H)-ones 2 in good yields (Scheme 1B). Herein, we would like to report our findings.
Kirsch and co-workers reported the synthesis of symmetric α,α-dioxoketene-N,S-acetals such as (methylthio(phenylamino)methylene)pentane-2,4-dione and diethyl 2-(methylthio(phenylamino)methylene)malonate.16 Therefore, according to the procedure described by Kirsch, we initially examined the preparation of 2-(ethylthio(phenylamino)methylene)-1-p-tolylbutane-1,3-dione 1a from one-pot reaction of 1-p-tolylbutane-1,3-dione, isorhodanides and bromoethane in the presence of K2CO3 at room temperature and found that 1a was obtained in 85% yield. The reaction showed high stereoselectivity and favored the formation of (Z)-1a which is confirmed by X-ray crystallographic analysis (Fig. 1).17 In a similar fashion, compounds 1b–k were prepared in excellent yields and with high stereoselectivity. Results of our findings are listed in Table 1. It is noteworthy that compounds 1 are stable at room temperature and could be stored indefinitely without decomposition.
| Entry | Ar | R | 1 | Yieldb (%) | Z/Ec |
|---|---|---|---|---|---|
| a Reaction conditions: 1-arylbutane-1,3-dione (5 mmol), isorhodanides (5 mmol), EtBr (5 mmol) and K2CO3 (10 mmol).b Isolated yields.c Molar ratio of Z/E-1 isomers determined by 1H NMR. | |||||
| 1 | 4-MeC6H4 | Ph | 1a | 85 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 3-MeC6H4 | Ph | 1b | 86 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 4-MeOC6H4 | Ph | 1c | 84 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Ph | Ph | 1d | 88 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 4-BrC6H4 | Ph | 1e | 90 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 2-Thiophenyl | Ph | 1f | 86 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Ph | 4-MeC6H4 | 1g | 83 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | Ph | 3-MeC6H4 | 1h | 85 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | Ph | 4-FC6H4 | 1i | 75 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | Ph | 4-BnC6H4 | 1j | 87 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | Ph | 2-MeC6H4 | 1k | 81 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
With α-acetyl-α-aroyl ketene-N,S-acetals 1 in hand, we started our investigation on Vilsmeier cyclization of compound 1 resulting in the formation of halogenated pyridin-2(1H)-ones. The reaction of 1a with Vilsmeier reagent POCl3/DMF was selected as model reaction to screen the experimental conditions. It was found that 1a reacted with Vilsmeier reagent POCl3/DMF (4 equiv.) below 70 °C to afford an unstable major product at room temperature (Table 2, entry 1–3). To our delight, when the reaction was carried out at 80 °C for 12 h, a stable white solid product was obtained in 35% yield. From the spectral and analytical data, the product was characterized as desirable product 4-chloro-5-(4-benzoyl)-6-oxo-1-phenyl-1,6-dihydropyridine-3-methylcarbaldehyde 2a (Table 2, entry 4). Additionally, the yield of 2a was not markedly improved by either prolonging reaction time or further elevating reaction temperature (Table 2, entry 5 and 6). However, the reaction showed dependence on the amount of Vilsmeier reagent POCl3/DMF (Table 2, entry 7–9), becoming more efficient with an increased load of this reagent. Consequently, 2a was obtained in 85% yield when 1a reacted with 6.0 equiv. of Vilsmeier reagent at 80 °C for 4 h (Table 2, entry 8). Accordingly, the optimal reaction conditions are 6.0 equiv. of Vilsmeier reagent and a temperature of 80 °C.
Next, we used the optimized reaction conditions to define the scope of this Vilsmeier cyclization for the synthesis of halogenated pyridin-2(1H)-ones, and the results are summarized in Table 3. The Vilsmeier cyclization of 1a–j and POCl3/DMF proceeded smoothly under the optimized conditions to efficiently afford corresponding 3-aroyl-4-chloro-5-formylpyridin-2(1H)-ones in high yields (Table 3, entry 1–10). It is noteworthy that the electronic effects of aromatic substituents on both aroyl and aryl amino groups are insignificant to the cyclization reaction. In the case of 2-((ethylthio)-(o-tolylamino)-methylene)-1-phenylbutane-1,3-dione 1k, 2k was isolated in 32% yield, which might stem from the steric hindered effect of the ortho-substituted methyl group on the benzene ring of aryl amine (Table 3, entry 11). However, when 1k reacted with POCl3/DMF (10 equiv.) at 120 °C for 12 h, the yield of 2k was enhanced up to 77% (Table 3, entry 11). The reaction was also successfully extended to the synthesis of 4-bromo pyridin-2(1H)-ones. According to Dong's work, the reaction of 1a and PBr3/DMF initially carried out under the optimized conditions afforded 4-bromo-5-(4-methylbenzoyl)-6-oxo-1-phenyl-1,6-dihydropyridine-3-carbaldehyde 2b in 21% yield (Table 3, entry 1). To our delight, when 1a reacted with Vilsmeier reagent POBr3/DMF under the same conditions, 2b was efficiently produced in 81% yield (Table 3, entry 1). Similarly, 1b–k underwent the Vilsmeier cyclization to efficiently afford the corresponding 3-aroyl-4-bromo-5-formyl pyridin-2(1H)-ones in high yields (Table 3, entry 2–11).
| Entry | 1 | Product 2 | Yieldb (%) | |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.25 mmol), POCl3/DMF or POBr3/DMF (1.5 mmol), 80 °C, 4–5 h.b Isolated yields.c The reaction of 1a and PBr3/DMF under the optimized conditions.d Reaction conditions: 1k (0.25 mmol), POCl3/DMF or POBr3/DMF (2.5 mmol), 120 °C, 12 h. | ||||
| 1 | 1a |  |
2a X = Cl | 85 |
| 2b X = Br | 21c | |||
| 81 | ||||
| 2 | 1b |  |
2c X = Cl | 82 |
| 2d X = Br | 80 | |||
| 3 | 1c |  |
2e X = Cl | 85 |
| 2f X = Br | 83 | |||
| 4 | 1d |  |
2g X = Cl | 84 |
| 2h X = Br | 84 | |||
| 5 | 1e |  |
2i X = Cl | 79 |
| 2j X = Br | 77 | |||
| 6 | 1f |  |
2k X = Cl | 80 |
| 2l X = Br | 79 | |||
| 7 | 1g |  |
2m X = Cl | 85 |
| 2n X = Br | 81 | |||
| 8 | 1h |  |
2o X = Cl | 82 |
| 2p X = Br | 83 | |||
| 9 | 1i |  |
2q X = Cl | 79 |
| 2r X = Br | 78 | |||
| 10 | 1j |  |
2s X = Cl | 85 |
| 2t X = Br | 85 | |||
| 11 | 1k |  |
2u X = Cl | 32 |
| 77d | ||||
| 2v X = Br | 74d | |||
Junjappa and co-workers investigated Vilsmeier reaction of α-acetyl ketene-N,S-acetals with POCl3/DMF (1.5 equiv.) at 80 °C and found that the diketonealdehyde enamine was obtained in moderate yield.18 In our research, we also checked the Vilsmeier reaction of α-acetyl ketene-N,S-acetals 1l under the above optimized conditions. Unlike Junjappa's work, the reaction furnished 4-chloro-1-(4-chlorophenyl)-2-oxo-1,2-dihydropyridine-3-carbaldehyde 2w in 61% yield (Scheme 2).
On the basis of the results obtained together with the relevant reports,9,10 a possible reaction mechanism is proposed in Scheme 3. It is clear that the reaction commenced from the halogenation of 1 in the presence of Vilsmeier reagent to generate vinyl chloride I. Sequential Vilsmeier reactions of I lead to the formation of intermediate II, which occurs intramolecular aza-cyclization reaction to give the intermediate III. Upon treatment with water, III is converted into the intermediate IV, which sheds dimethylamine to give the intermediate V. Finally, halogenated pyridine-2(1H)-ones 2 are obtained after the removal of H proton.
Conclusion
In conclusion, we firstly prepared (Z)-α-acetyl-α-aroyl ketene-N,S-acetals 1 and confirmed their configuration by X-ray crystallographic analysis. Subsequently, a facile and efficient one-pot synthesis of 3-aroyl-5-formyl-4-halopyridin-2(1H)-ones 2 was successfully developed from Vilsmeier cyclizations of readily available substrate 1. Further application of synthesized halogenated pyridin-2(1H)-ones 2 is ongoing in our group.Acknowledgements
We are grateful to the National Nature Science Foundation of China (20902010) and Science Foundation of Anshan City (2011MS44, 2012KJ02, 2014KJ05) for support of this research. Many Thanks are due to Professor XiHe Bi for his critical reading of the manuscript.Notes and references
- (a) A. D. Elbein and R. J. Molyneux, in Alkaloids: Chemical and Biological Perspectives, ed. S. W. Pelletier, Wiley, New York, 1981, vol. 5, pp. 1–54 Search PubMed; (b) Q. Li, L. A. Mitscher and L. L. Shen, Med. Res. Rev., 2000, 20, 231–293 CrossRef CAS PubMed; (c) J. A. Varela and C. Saa, Chem. Rev., 2003, 103, 3787–3801 CrossRef CAS PubMed; (d) L. M. José, M. M. Karina, J. G. Cecilia and C. Rafael, ChemMedChem, 2007, 2, 1141–1147 CrossRef PubMed; (e) W. S. Hamama, M. Waly, I. El-Hawary and H. H. Zoorob, Synth. Commun., 2014, 44, 1730–1759 CrossRef CAS.
- (a) I. R. Baxendale, S. V. Ley and C. Piutti, Angew. Chem., Int. Ed., 2002, 41, 2194–2197 CrossRef CAS; (b) L. M. Halo, M. N. Heneghan, A. A. Yakasai, Z. S. Song, K. Williams, A. M. Bailey, R. J. Cox, C. M. Lazarus and T. J. Simpson, J. Am. Chem. Soc., 2008, 130, 17988–17996 CrossRef CAS PubMed; (c) J. Guillemont, A. Benjahad, S. Oumouch, L. Decrane, P. Palandjian, D. Vernier, L. Queguiner, K. Andries, M. P. Béthune, K. Hertogs, D. S. Grierson and C. H. Nguyen, J. Med. Chem., 2009, 52, 7473–7487 CrossRef CAS PubMed; (d) A. Li, Y. B. Ouyang, Z. Y. Wang, Y. Y. Cao, X. Y. Liu, L. Ran, C. Li, L. Li, L. Zhang, K. Qiao, W. S. Xu, Y. Huang, Z. L. Zhang, C. Tian, Z. M. Liu, S. B. Jiang, Y. M. Shao, Y. S. Du, L. Y. Ma, X. W. Wang and J. Y. Liu, J. Med. Chem., 2013, 56, 3593–3608 CrossRef CAS PubMed; (e) A. Haga, H. Tamoto, M. Ishino, E. Kimura, T. Sugita, K. Kinoshita, K. Takahashi, M. Shiro and K. Koyama, J. Nat. Prod., 2013, 76, 750–754 CrossRef CAS PubMed.
- (a) J. M. Domagala, J. Heterocycl. Chem., 1984, 21, 1705–1708 CrossRef CAS; (b) J. K. Stille, Angew. Chem., Int. Ed., 1986, 25, 508–524 CrossRef; (c) R. Kawai, M. Kimoto, S. Ikeda, T. Mitsui, M. Endo, S. Yokoyama and I. Hirao, J. Am. Chem. Soc., 2005, 127, 17286–17295 CrossRef CAS PubMed; (d) Z. Sun, S. Ahmed and L. W. Mclaughlin, J. Org. Chem., 2006, 71, 2922–2925 CrossRef CAS PubMed; (e) D. Conreaux, E. Bossharth, N. Monteiro, P. Desbordes, J. P. Vors and G. Balme, Org. Lett., 2007, 9, 271–274 CrossRef CAS PubMed.
- (a) I. N. Houpis, W. B. Choi, P. J. Reider, O. Molina, H. Churchill, J. Lynch and R. P. Volante, Tetrahedron Lett., 1994, 35, 9355–9358 CrossRef CAS; (b) W. B. Choi, I. N. Houpis, H. Churchill, O. Molina, J. E. Lynch, R. P. Volante, P. J. Reider and A. O. King, Tetrahedron Lett., 1995, 36, 4571–4574 CrossRef CAS.
- (a) R. A. Abramovitch and J. G. Saha, Adv. Heterocycl. Chem., 1966, 6, 229–231 CrossRef CAS; (b) O. S. Tee and M. Paventi, J. Am. Chem. Soc., 1982, 104, 4142–4146 CrossRef CAS.
- J. F. Marcoux, F. A. Marcotte, J. Wu, P. G. Dormer, I. W. Davies, D. Hughes and P. J. Reider, J. Org. Chem., 2001, 66, 4194–4199 CrossRef CAS PubMed.
- (a) C. Agami, L. Dechoux, D. Hebbe and J. Moulinas, Synthesis, 2002, 79–82 CrossRef CAS; (b) J. Adams, A. Hardin and F. Vounatsos, J. Org. Chem., 2006, 71, 9895–9898 CrossRef CAS PubMed.
- X. W. Wang, H. F. Cui, H. F. Wang, Y. Q. Yang, G. Zhao and S. Z. Zhu, Tetrahedron, 2011, 67, 2468–2473 CrossRef CAS.
- Y. J. Liang, P. Huang, R. Zhang and D. W. Dong, Chin. J. Org. Chem., 2014, 34, 1037–1047 CrossRef CAS.
- (a) W. Pan, D. Dong, K. Wang, J. Zhang, R. Wu, D. Xiang and Q. Liu, Org. Lett., 2007, 9, 2421–2423 CrossRef CAS PubMed; (b) L. Chen, Y. L. Zhao, Q. Liu, C. Cheng and C. R. Piao, J. Org. Chem., 2007, 72, 9259–9263 CrossRef CAS PubMed; (c) D. Xiang, Y. Yang, R. Zhang, Y. Liang, W. Pan, J. Huang and D. Dong, J. Org. Chem., 2007, 72, 8593–8596 CrossRef CAS PubMed; (d) D. Xiang, K. Wang, Y. Liang, G. Zhou and D. Dong, Org. Lett., 2008, 10, 345–348 CrossRef CAS PubMed; (e) R. Zhang, D. Y. Zhang, Y. L. Guo, G. Y. Zhou, Z. J. Jiang and D. W. Dong, J. Org. Chem., 2008, 73, 9504–9507 CrossRef CAS PubMed.
- (a) R. K. Dieter, Tetrahedron, 1986, 42, 3029–3096 CrossRef CAS; (b) H. Junjappa, H. Ila and C. V. Asokan, Tetrahedron, 1990, 46, 5423–5506 CrossRef CAS; (c) L. Pan and Q. Liu, Synlett, 2011, 1073–1080 CAS; (d) L. Pan, X. H. Bi and Q. Liu, Chem. Soc. Rev., 2013, 42, 1251–1286 RSC.
- (a) S. Chakrabarti, K. Panda, N. C. Misra, H. Ila and H. Junjappa, Synlett, 2005, 1437–1441 CAS; (b) R. T. Chakrasali and H. Junjappa, Synthesis, 1988, 87–89 CrossRef CAS; (c) A. K. Gupta, R. T. Chakrasali, H. Ila and H. Junjappa, Synthesis, 1989, 141–142 CrossRef CAS; (d) D. Pooranchand, H. Ila and H. Junjappa, Synthesis, 1987, 547–550 CrossRef CAS; (e) A. Rahman, H. Ila and H. Junjappa, J. Chem. Soc., Chem. Commun., 1984, 430–431 RSC.
- For our recent work on ketene-S,S-acetals as thiol equivalents, see: (a) H. F. Yu, D. L. Wang, H. Zhao and D. Y. Hou, Chin. J. Org. Chem., 2011, 31, 1949–1960 CAS; (b) H. F. Yu, Chin. J. Chem., 2012, 23, 367–371 CrossRef; (c) H. F. Yu and P. Q. Liao, Chem. J. Chin. Univ., 2012, 33, 1969–1972 CAS; (d) H. F. Yu, Synth. Commun., 2013, 43, 1280–1986 CrossRef CAS; (e) H. F. Yu and P. Q. Liao, Chemistry, 2013, 5, 435–437 Search PubMed.
- For our recent work on the C–H bond activation on ketene-S,S-acetals, see: (a) H. F. Yu, W. W. Jin, C. L. Sun and Z. K. Yu, Angew. Chem., Int. Ed., 2010, 49, 5792–5797 CrossRef CAS PubMed; (b) Z. F. Mao, F. Huang, H. F. Yu, J. P. Chen, Z. K. Yu and Z. Q. Xu, Chem.–Eur. J., 2014, 20, 3439–3445 CrossRef CAS PubMed.
- For our recent work on the functionalization of indoles based on ketene-S,S-acetals, see: (a) H. F. Yu and Z. K. Yu, Angew. Chem., Int. Ed., 2009, 48, 2929–2933 CrossRef CAS PubMed; (b) W. W. Jin, W. M. Du, Q. Yang, H. F. Yu, J. P. Chen and Z. K. Yu, Org. Lett., 2011, 13, 4272–4275 CrossRef CAS PubMed; (c) W. W. Jin, H. F. Yu and Z. K. Yu, Tetrahedron Lett., 2011, 52, 5884–5887 CrossRef CAS; (d) H. F. Yu, T. C. Li and P. Q. Liao, Synthesis, 2012, 3743–3756 CrossRef CAS; (e) H. F. Yu, T. C. Li, P. Q. Liao, G. Xin and D. Y. Hou, Chin. J. Org. Chem., 2014, 34, 956–959 CrossRef CAS; (f) H. F. Yu, P. Q. Liao, Q. P. Diao, T. C. Li, G. Xin, L. N. Han and D. Y. Hou, Chin. J. Org. Chem., 2014, 34, 1851–1856 CrossRef CAS.
- (a) G. Sommen, A. Comel and G. Kirsch, Synlett, 2001, 1731–1734 CrossRef CAS; (b) G. Sommen, A. Comel and G. Kirsch, Tetrahedron Lett., 2002, 43, 257–259 CrossRef CAS.
- ESI†.
- P. K. Mahata, C. Venkatesh, U. K. Syam Kumar, H. Ila and H. Junjappa, J. Org. Chem., 2003, 68, 3966–3975 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical data, and spectra copies of all compounds. CCDC 974611. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra14626c |
| This journal is © The Royal Society of Chemistry 2015 |