Rhodamine derived colorimetric and fluorescence mercury(II) chemodosimeter for human breast cancer cell (MCF7) imaging†
Babli Kumaria,
Sisir Lohara,
Sangita Adhikaria,
Archya Sengupta b,
Ansuman Chattopadhyayb,
Paula Brandãoc,
Vítor Félix*d and
Debasis Das*a
b,
Ansuman Chattopadhyayb,
Paula Brandãoc,
Vítor Félix*d and
Debasis Das*a
aDepartment of Chemistry, The University of Burdwan, Burdwan, West Bengal, India. E-mail: ddas100in@yahoo.com; Fax: +91-342-2530452; Tel: +91-342-2533913
bDepartment of Zoology, Visva Bharati University, Santiniketan, West Bengal, India
cDepartamento de Química, CICECO, Universidade de Aveiro, 3810-193 Aveiro, Portugal
dDepartamento de Química, CICECO and Secção Autónoma de Ciências da Saúde, Universidade de Aveiro, 3810-193 Aveiro, Portugal
First published on 17th February 2015
Abstract
Condensation of rhodaminehydrazone with naphthalene-1-carboxaldehyde generates a colourless probe, RDHDNAP that can selectively detect Hg2+ through generation of a pink color along with significant fluorescence enhancement. The binding constant and lowest detection limit for Hg2+ are 2.0 × 105 M−1 and 3 × 10−7 M respectively. Hg2+ imaging in human breast cancer cells (MCF7) under a fluorescence microscope is achieved.
Mercury, one of the heavy metals, is widely used in different industries1 and has several other sources like volcanic emissions, gold mining, and solid waste incineration.2 It can easily permeate biological membranes such as the skin and gastrointestinal tissues. Being thiophilic in nature, it has high affinity for proteins and enzymes3 and causes neurological problems,4 myocardial infarction,5 DNA damage, Minamata disease,6 and other chronic diseases.7 The US Environmental Protection Agency (USEPA) has recommended 2 ppb inorganic Hg2+ as maximum tolerance level in drinking water.8 Hence, ultra-trace level selective determination of Hg2+ at physiological condition is very demanding.9 Conventional available techniques include atomic absorption/emission spectroscopy, neutron activation analysis, inductively coupled plasma mass spectrometry, cold vapour atomic fluorescence spectrometry, electrochemical sensing and X-ray microanalysis etc. However, methods based on absorption/emission spectroscopy have several advantages, viz. fast, non-destructive, economic, user friendly involving facile sample preparation process. Several known photo sensing processes include photo induced electron transfer (PET),10 photo-induced charge transfer (PCT),11 fluorescence resonance energy transfer (FRET),12 intermolecular charge transfer (ICT),13 chelation enhanced fluorescence (CHEF),14 perturbation of optical transitions and polarisabilities and intra-molecular charge transfer (ICT).15 Due to spin orbit coupling, Hg2+ generally quenches fluorescence upon binding to a probe. Amongst several Hg2+ selective fluorescence sensors,16 rhodamine based probes have several advantages like large absorption coefficient, high fluorescence quantum yield, absorption and emission in the visible region.17,18 The spirolactam form of rhodamine-B is colourless with an absorption band ∼250 nm that undergoes significant fluorescence enhancement along with colour generation in presence of specific analyte(s), attributed to the spirolactam ring opening.19 Choice of solvent plays a crucial role for biological studies and green analysis. Most of the rhodamine-based Hg2+ sensors function in CH3CN–H2O solution,20 toxic to the living system, while several others used in EtOH–H2O (2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).21 The present probe functions in much greener media, EtOH–H2O (3
1, v/v).21 The present probe functions in much greener media, EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v). The facile synthesis, structural confirmation by single crystal X-ray crystallography and functioning in a greener solvent place the present probe (RDHDNAP) in an advantageous position for colorimetric and fluorescence recognition of intra-cellular Hg2+ in human breast cancer cell (MCF7).
7, v/v). The facile synthesis, structural confirmation by single crystal X-ray crystallography and functioning in a greener solvent place the present probe (RDHDNAP) in an advantageous position for colorimetric and fluorescence recognition of intra-cellular Hg2+ in human breast cancer cell (MCF7).
Results and discussion
Single crystals of RDHDNAP (1) and its model compound (2) containing pyrene are grown from their ethanol solution and characterized by X-ray crystallography. The most significant structural data are listed in Table 1. The molecular diagram of 1, presented in Fig. 1 (top) shows the formation of spirolactam moiety having hydrazone functional group. The bond lengths and angles subtended at the spiro atom are consistent with the existence of quaternary carbon centre. In agreement, the spirolactam and xanthene rings adopt almost an orthogonal arrangement with an Ω angle between their least square planes being 85.15(2)°. The formation of the N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C functional group is corroborated by N(32)–N(34) and N(34)–C(35) bond lengths with values of 1.376(2) and 1.281(2) Å, respectively.
C functional group is corroborated by N(32)–N(34) and N(34)–C(35) bond lengths with values of 1.376(2) and 1.281(2) Å, respectively.
| Compound | 1 | 2 |
|---|---|---|
| C(7)–C(8) | 1.519(2) | 1.509(3) |
| C(8)–C(9) | 1.517(2) | 1.524(3) |
| C(8)–C(25) | 1.527(2) | 1.522(4) |
| C(8)–N(32) | 1.494(2) | 1.506(3) |
| N(32)–N(34) | 1.376(2) | 1.389(3) |
| N(34)–C(35) | 1.281(2) | 1.272(4) |
| C(31)–N(32) | 1.386(2) | 1.391(3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| C(7)–C(8)–C(25) | 110.64(12) | 111.6(2) |
| C(9)–C(8)–C(7) | 109.82(12) | 110.91(19) |
| C(9)–C(8)–C(25) | 111.43(14) | 111.5(2) |
| N(32)–C(8)–C(7) | 112.44(13) | 111.37(19) |
| N(32)–C(8)–C(9) | 112.65(12) | 111.38(19) |
| N(32)–C(8)–C(25) | 99.52(12) | 99.61(19) |
| C(31)–N(32)–C(8) | 114.07(13) | 114.0(2) |
| N(34)–N(32)–C(8) | 127.55(12) | 127.9(2) |
| N(34)–N(32)–C(31) | 117.25(14) | 117.9(2) |
| C(35)–N(34)–N(32) | 118.14(14) | 119.7(2) |
In the crystal structure, pairs of RDHDNAP molecules are self-assembled in a centro-symmetric arrangement, stabilized by π⋯π stacking interactions with an inter-planar and shift distances between the naphthalene rings of 3.714(1) and 1.336(2) Å, respectively. In addition, two C–H⋯π intermolecular edge-to-face interactions with distances of 2.49 Å have been observed between both partners, along with two intra-molecular ones with distances of and 2.77 Å, as illustrated in Fig. 1 (bottom).
Effect of pH on the emission characteristics of RDHDNAP and its Hg2+ adduct has been examined. Fig. 2 reveals higher emission intensity of free RDHDNAP at lower pH, attributed to spirolactam ring opening. In presence of Hg2+, RDHDNAP shows higher emission intensity at pH 6 to 10, enabling its detection and estimation at physiological pH. Addition of Hg2+ to colourless RDHDNAP causes instant pink colouration, along with appearance of 558 nm peak due to Hg2+ induced ring-opening of the probe (Fig. 3). This new peak gradually increases upon incremental addition of Hg2+.
 | ||
| Fig. 2 Effect of pH on emission intensity of RDHDNAP (20 μM) (black) and RDHDNAP–Hg2+ system (red) in HEPES buffered (0.1 M) solution (ethanol–water = 3/7, v/v, pH 7.4); λex = 550 nm, λem = 591 nm. | ||
 | ||
| Fig. 3 Changes in the absorption spectra of RDHDNAP (20 μM) upon gradual addition of Hg2+ (1.0, 2.5, 7.5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 400, 600 and 800 μM); inset: naked eye colour of free RDHDNAP and [RDHDNAP–Hg2+] system. Same solvent media as noted in Fig. 2 caption. | ||
Free RDHDNAP exhibits a very weak emission at 591 nm (λex = 550 nm) characteristics of its close spirolactam ring structure which gradually increases upon gradual addition of Hg2+ (Fig. 4). This is attributed to Hg2+ assisted hydrolysis of RDHDNAP (Fig. 5), corroborated from the mass spectrum of the end product (Fig. S1, ESI†). Initially, Hg2+ assisted hydrolysis of RDHDNAP leads to ring opening, followed by rapture of imine bond. Further, hydrazine unit is dislodged yielding free open ring rhodamine-B unit.
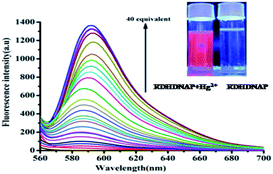 | ||
| Fig. 4 Changes in the emission spectra of RDHDNAP (20 μM) upon gradual addition of Hg2+ (0.1, 0.2, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 25.0, 50.0, 100.0, 200.0, 400.0, 600.0 and 800.0 μM, λex = 550 nm). Inset: corresponding UV light exposed colour change. Same solvent media as noted in Fig. 2 caption. | ||
In presence of Hg2+, fluorescence quantum yield of RDHDNAP increases 55.6 fold (from 0.0079 to 0.44) (ESI). Plot of emission intensities vs. added Hg2+ concentration is sigmoidal (Fig. S2, ESI†). Other bio-relevant and common cations viz. Mn2+, Cd2+, Ni2+, Co2+, Mg2+, Fe3+, Hg2+, Cu2+, Ca2+, Pb2+, Zn2+, Fe2+ and Pb2+ fail to cause any significant colour change of RDHDNAP, either naked eye or under UV light (Fig. 6).
Probably, due to strong paramagnetic effect, none other than Cu2+ interfere a little (Fig. 7). Job's plot (Fig. S3, ESI†) shows 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mole ratio) binding interaction between RDHDNAP and Hg2+. The association constant obtained following Benesi–Hildebrand method22 is 2.0 × 105 M−1 (Fig. S4, ESI†). RDHDNAP can detect as low as 3 × 10−7 M Hg2+, very much competitive to the reported values for other rhodamine based Hg2+ sensors useful for cell imaging studies, and listed in tabular form in ESI.†
1 (mole ratio) binding interaction between RDHDNAP and Hg2+. The association constant obtained following Benesi–Hildebrand method22 is 2.0 × 105 M−1 (Fig. S4, ESI†). RDHDNAP can detect as low as 3 × 10−7 M Hg2+, very much competitive to the reported values for other rhodamine based Hg2+ sensors useful for cell imaging studies, and listed in tabular form in ESI.†
 | ||
| Fig. 7 Relative emission intensities of RDHDNAP + Hg2+ system in presence of different cations: [RDHDNAP + Hg2+] (black); RDHDNAP–Hg2+ + cations (100 eq.) (red); λex = 550 nm, λem = 591 nm. Same solvent media as noted in Fig. 2 caption. | ||
Although, Yao et al. have reported its analogues pyrene derivative (2)23 as Hg2+ sensor, however, the performance of the present probe, RDHDNAP is better in terms of LOD (100 times lower). Additionally, Hg2+ imaging in human breast cancer cell (MCF7) is possible with RDHDNAP. Fig. 8 shows the single crystal X-ray structure of 2. The bond lengths and bond angles around the spiro carbon centre as well as for the hydrazine functional group are similar to those found for 1. The most relevant structural feature in the crystal packing of the pyrene analogue is the formation of dimeric self-assembled molecules assisted by C–H⋯π stacking interactions between extended pyrene rings at an inter-planar distance of 3.998(2) Å, and shitted by 1.981(3) Å. Furthermore, two molecular entities adopt a ladder type spatial disposition (Fig. 8, bottom).
Due to poor water solubility, intra-cellular Hg2+ imaging using the pyrene probe (1) remains unsuccessful.
Human breast cancer cell imaging
Human breast cancer cells (MCF7) treated only with RDHDNAP, (without Hg2+) are used as control. RDHDNAP can detect intracellular Hg2+ very efficiently (Fig. 9).The application of this new method is substantiated by analyzing Hg2+ content in several industrial waste water samples and comparing the results obtained from a reference method24 (Table 2). The close proximity of the results of a certified reference material by the present method clearly indicates its acceptability for Hg2+ determination in real samples.
| Sample | Hg2+ founda (μg g−1, present method) | Hg2+ found (μg g−1, reference method) |
|---|---|---|
| a Average value, N = 3.b NIES certified value: 1.2 ± 0.2 μg g−1. | ||
| Industrial waste water (station 1) | 141.2 ± 0.2 | 140.7 ± 0.4 |
| Industrial waste water (station 2) | 179.2 ± 0.4 | 179.8 ± 0.2 |
| Industrial waste water (station 3) | 165.2 ± 0.4 | 165.6 ± 0.3 |
| Industrial waste water (station 4) | 225.7 ± 0.6 | 226.4 ± 0.6 |
| NIES certified sampleb | 1.15 ± 0.2 | 1.14 ± 0.2 |
Conclusion
Single crystal X-ray structurally characterised rhodaminehydrazone derivative, RDHDNAP is an excellent colorimetric and fluorescence probe for Hg2+ at physiological pH. Its Hg2+ detection limit is 3 × 10−7 M. RDHDNAP senses Hg2+ via hydrolysis mechanism, useful to monitor intracellular Hg2+ accumulation in human breast cancer cell.Experimental
General procedure
Rhodamine B, naphthalene-1-carboxaldehyde and pyrene-1-carboxaldehyde have been purchased from Sigma Aldrich (India). Spectroscopic grade solvents have been used. Other chemicals are of analytical reagent grade and used without further purification. Milli-Q 18.2 MΩ cm−1 water has been used as and when required. Shimadzu UV-Vis-2450 spectrophotometer has been used for measuring the absorption spectra. FTIR spectra are recorded on a Shimadzu FTIR spectrometer. Mass spectra are obtained using QTOF Micro YA 263 mass spectrometer in ES positive mode. 1H NMR spectra have been recorded using Bruker Advance 300 (300 MHz) instrument in CDCl3. Steady-state fluorescence experiments have been performed using Hitachi F-4500 spectrofluorimeter. The pH of the solutions has been measured using Systronics digital pH meter (model 335, India). The industrial waste water samples have been collected from Damodar river (Durgapur-Raniganj Industrial belt, West Bengal, India), filtered through 0.45 μm Millipore membrane filter and analyzed using RDHDNAP.Synthesis of rhodaminehydrazone unit
N-(Rhodamine-B) lactam-hydrazine is prepared by refluxing Rhodamine B and hydrazine hydrate for 3 h following literature method.25![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) 1585 (Fig. S9, ESI†).
O) 1585 (Fig. S9, ESI†).X-ray crystallography
The single crystal X-ray data of RDHDNAP (1) and analogous pyrene derivative (2) are collected with monochromated Mo-Kα radiation (λ = 0.71073 Å) on a Bruker SMART Apex II diffractometer equipped with a CCD area detector at 150(2) K and at room temperature, respectively. The crystal is positioned at 35 mm from the CCD and the spots are measured using counting time of 80 and 100 s for 1 and 2, respectively. Data reduction is carried out using the SAINT-NT software package.26 A multi-scan absorption correction is applied to all intensity data of each compound using the SADABS program.27 Both the structures are solved by combination of direct methods with subsequent difference Fourier syntheses and refined by full matrix least squares on F2 using the SHELX-2013 suite.28 Hydrogen atoms bound to carbon are inserted at ideal geometric positions. Molecular and the crystal packing diagrams are drawn with the PyMOL software package.29 The crystal data together with refinement details are given in Table 3.| Complex | 1 | 2 |
|---|---|---|
| Empirical formula | C39H38N4O2 | C45H40N4O2 |
| Mw | 594.73 | 668.81 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21/c |
| a [Å] | 11.5411(14) | 9.273(4) |
| b [Å] | 11.5412(10) | 16.808(7) |
| c [Å] | 23.608(2) | 23.315(9) |
| β [°] | 92.592(4) | 100.688(15) |
| V [Å3] | 3141.4(6) | 3571(2) |
| Z | 4 | 4 |
| Dc [Mg m−3] | 1.258 | 1.244 |
| μ [mm−1] | 0.078 | 0.077 |
| Reflections collected | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 045 |
| Unique reflections, [Rint] | 6437 [0.0366] | 7267 [0.0788] |
| Final R indices | ||
| R1, wR2 [I > 2σI] | 0.0456, 0.1027 [4432] | 0.0673, 0.1798 [3736] |
| R1, wR2 (all data) | 0.0760, 0.1163 | 0.1297, 0.2194 |
Cytotoxicity assay
In vitro cytotoxicity is measured using the colorimetric methyl thiazolyl tetrazolium (MTT) assay against human breast cancer cells (MCF7). Cells are seeded into 24-well tissue culture plate in presence of 500 μL Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C and 5% CO2 atmosphere for overnight and then incubated for 12 h in presence of RDHDNAP at different concentrations (10–100 μM). Then cells are washed with PBS buffer and 500 μL supplemented DMEM medium is added. Subsequently, 50 μL MTT (5 mg mL−1) is added to each well and incubated for 4 h. Next, violet formazan is dissolved in 500 μL sodium dodecyl sulfate solution in water–DMF mixture. The absorbance of solution is measured at 558 nm using microplate reader. The cell viability is determined by assuming 100% cell viability for cells without RDHDNAP (Fig. 10).In vitro cell imaging
Human breast cancer cell line, MCF7 is grown in DMEM (Sigma, St. Louis, USA) supplemented with 10% fetal bovine serum (Sigma, St. Louis, USA), 2 mM glutamine, 100 U mL−1 penicillin–streptomycin solution (Gibco, Invitrogen, USA) in the presence of 5% CO2 at 37 °C. For in vitro imaging study, the cells are seeded in 6 well culture plate with a seeding density of 105 cells per well. After reaching 60–70% confluence, the previous complete media is replaced with serum free media, supplemented with Hg2+ and RDHDNAP at a concentration of 50 and 20 μM followed by incubation for 2 h to facilitate uptake of cation or RDHDNAP by cells. The cells are then observed under an inverted microscope (Dewinter, Italy) at different magnifications to examine any adverse effect on cellular morphology. RDHDNAP treated cells are then incubated with Hg2+ for 15–30 min and observed under an inverted fluorescence microscope (Dewinter, Italy) at different magnifications using blue filter. Images are taken through an attached CCD camera with the help of BioWizard 4.2 software. Control experiment is performed using the medium devoid of metal salt.Acknowledgements
Authors sincerely thank UGC-DAE-CRS-Kolkata for financial support. B. Kumari sincerely thanks BU for fellowship. S. Lohar is thankful to CSIR for fellowship. Authors sincerely acknowledge CAS (B. U.) for assistance. Suggestions from reviewers to improve the quality of the MS are gratefully acknowledged.Notes and references
- (a) Q. Wang, D. Kim, D. D. Dionysiou, G. A. Sorial and D. Timberlake, Environ. Pollut., 2004, 131, 323 CrossRef CAS PubMed; (b) E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS PubMed; (c) C. M. L. Carvalho, E.-H. Chew, S. I. Hashemy, J. Lu and A. Holmgren, J. Biol. Chem., 2008, 283, 11913 CrossRef CAS PubMed; (d) G. Guzzi and C. A. M. La Porta, Toxicology, 2008, 244, 1 CrossRef CAS PubMed.
- (a) R. Von Burg and M. R. Greenwood, Metals, VCH, Weinheim, 1991, p. 1045 Search PubMed; (b) A. Renzoni, F. Zino and E. Franchi, Environ. Res., 1998, 77, 68 CrossRef CAS PubMed; (c) K. Rurack, M. Kollmannsberger, U. Resch-Genger and J. Dauhb, J. Am. Chem. Soc., 2000, 122, 968 CrossRef CAS.
- (a) B. R. Von, J. Appl. Toxicol., 1995, 15, 483 CrossRef; (b) H. H. Harris, I. J. Pickering and G. N. George, The Chemical Form of Mercury in Fish, Science, 2003, 301, 1203 CrossRef CAS PubMed.
- J. Gutknecht, J. Membr. Biol., 1981, 61, 61 CrossRef CAS.
- G. N. George, R. C. Prince, J. Gailer, G. A. Buttigieg, M. B. Denton, H. H. Harris and I. J. Pickering, Chem. Res. Toxicol., 2004, 17, 999 CrossRef CAS PubMed.
- M. Harada, Crit. Rev. Toxicol., 1995, 25, 1 CrossRef CAS PubMed.
- G. D. Huy, M. Zhang, P. Zuo and B.-C. Ye, Analyst, 2011, 136, 3289 RSC.
- Mercury Update: Impact on Fish Advisories. EPA Fact Sheet EPA-823-F-01-011, EPA, Office of Water, Washington, DC, 2001.
- (a) Z. Xu, S. J. Han, C. Lee, J. Yoon and D. R. Spring, Chem. Commun., 2010, 46, 1679 RSC; (b) M. Suresh, S. Mishra, S. K. Mishra, E. Suresh, A. K. Mandal, A. Shrivastav and A. Das, Org. Lett., 2009, 11, 2740 CrossRef CAS PubMed.
- J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780 CrossRef CAS PubMed.
- (a) S. K. Kim, J. H. Bok, R. A. Bartsch, J. Y. Lee and J. S. Kim, Org. Lett., 2005, 7, 4839 CrossRef CAS PubMed; (b) H. J. Kim, S. K. Kim, J. Y. Lee and J. S. Kim, J. Org. Chem., 2006, 71, 6611 CrossRef CAS PubMed.
- (a) B. W. van der Meer, G. Coker III and S. Y. Simon, Resonance Energy Transfer, Theory and Data, VCH, Weinheim, 1994 Search PubMed; (b) I. Leray, F. O'Reilly, J. L. Habib Jiwan, J. P. Soumillion and B. Valeur, Chem. Commun., 1999, 795 RSC; (c) B. Liu, F. Zeng, G. Wu and S. Wu, Chem. Commun., 2011, 47, 8913 RSC; (d) R. K. Castellano, S. L. Craig, C. Nuckolls and J. Rebek, J. Am. Chem. Soc., 2000, 122, 7876 CrossRef CAS.
- (a) Z. Xu, Y. Xiao, X. Qian, J. Cui and D. Cui, Org. Lett., 2005, 7, 889 CrossRef CAS PubMed; (b) J. B. Wang, X. F. Qian and J. N. Chi, J. Org. Chem., 2006, 71, 4308 CrossRef CAS PubMed.
- (a) N. C. Lim, J. V. Schuster, M. C. Porto, M. A. Tanudra, L. Yao, H. C. Freake and C. Bruckner, Inorg. Chem., 2005, 44, 2018 CrossRef CAS PubMed; (b) S. Guha, S. Lohar, A. Banerjee, A. Sahana, A. Chaterjee, S. K. Mukherjee, J. S. Matalobos and D. Das, Talanta, 2012, 91, 18 CrossRef CAS PubMed; (c) S. Das, A. Sahana, A. Banerjee, S. Lohar, S. Guha, J. S. Matalobos and D. Das, Anal. Methods, 2012, 4, 2254 RSC.
- S. A. de Silva, A. Zavaleta, D. E. Baron, O. Allam, E. V. Isidor, N. Kashimura and J. M. Percapio, Tetrahedron Lett., 1997, 38, 2237 CrossRef CAS.
- (a) A. Kaur, K. Sharma, S. Kaur, N. Singh and N. Kaur, RSC Adv., 2013, 3, 6160 RSC; (b) B. N. Ahmed and P. Ghosh, Dalton Trans., 2011, 40, 12540 RSC.
- (a) S. Adhikari, A. Ghosh, S. Mandal, A. Sengupta, A. Chattopadhyay, J. S. Matalobos, S. Lohar and D. Das, Dalton Trans., 2014, 43, 7747 RSC; (b) S. Mandal, A. Banerjee, S. Lohar, A. Chattopadhyay, B. Sarkar, S. K. Mukhopadhyay, A. Sahana and D. Das, J. Hazard. Mater., 2013, 261, 198 CrossRef CAS PubMed; (c) M. J. Culzoni, A. Muňoz de la Peňa, A. Machuca, H. C. Goicoechea and R. Babiano, Anal. Methods, 2013, 5, 30 RSC.
- (a) A. Banerjee, A. Sahana, S. Lohar, I. Halui, S. K. Mukhopadhyay, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia and D. Das, Chem. Commun., 2013, 49, 2527 RSC; (b) B. Bag and A. Pal, Org. Biomol. Chem., 2011, 9, 4467 RSC; (c) X. Guan, W. Lin and W. Huang, Org. Biomol. Chem., 2014, 12, 3944 RSC.
- (a) H. Wang, Y. Li, S. Xu, Y. Li, C. Zhou, X. Fei, L. Sun, C. Zhang, Y. Li, Q. Yang and X. Xu, Org. Biomol. Chem., 2011, 9, 2850 RSC; (b) X. Zhang, X. J. Huang and Z. J. Zhu, RSC Adv., 2013, 3, 24891 RSC.
- (a) J. H. Soh, K. M. K. Swamy, S. K. Kim, S. Kim, S. H. Lee and J. Yoon, Tetrahedron Lett., 2007, 48, 5966 CrossRef CAS PubMed; (b) Y. Zhou, K. Chu, H. Zhen, Y. Fang and C. Yao, Spectrochim. Acta, Part A, 2013, 106, 197 CrossRef CAS PubMed; (c) H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC.
- (a) Y. Zhou, X. Y. You, Y. Fang, J. Y. Li, K. Liu and C. Yao, Org. Biomol. Chem., 2010, 8, 4819 RSC; (b) Z. Zhou, M. Yu, H. Yang, K. Huang, F. Li, T. Yi and C. Huang, Chem. Commun., 2008, 3387 RSC; (c) H. Yang, Z. Zhou, K. Huang, M. Yu, F. Li, T. Yi and C. Huang, Org. Lett., 2007, 9, 4729 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- K. H. Chu, Y. Zhou, Y. Fang, L. H. Wang, J. Y. Li and C. Yao, Dyes Pigm., 2013, 98, 339 CrossRef CAS PubMed.
- E. M. Soliman, M. B. Saleh and S. A. Ahmed, Anal. Chim. Acta, 2004, 523, 133 CrossRef CAS PubMed.
- X. F. Yang, X. Q. Guo and Y. B. Zhao, Talanta, 2002, 57, 883 CAS.
- Bruker SAINT-plus, Bruker AXS Inc., Madison, Wisconsin, USA, 2007 Search PubMed.
- G. M. Sheldrick, SADABS, University of Göttingen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- The PyMOL Molecular Graphics System, Version 1.5.0.4, Schrödinger, LLC Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 961580 and 900558. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra14624g |
| This journal is © The Royal Society of Chemistry 2015 |







