Tumor-targeted co-delivery of mitomycin C and 10-hydroxycamptothecin via micellar nanocarriers for enhanced anticancer efficacy†
Jinyan Linad,
Yang Liacd,
Hongjie Wub,
Xiangrui Yangad,
Yanxiu Lia,
Shefang Yea,
Zhenqing Hou*a and
Changjian Linc
aDepartment of Biomaterials, Research Center of Biomedical Engineering, Institute of Soft Matter and Biomimetics, College of Materials, Xiamen University, Xiamen 361005, China. E-mail: houzhenqing@xmu.edu.cn; Fax: +86-592-2183508; Tel: +86-592-2186178
bDepartment of Pharmacy, School of Pharmaceutical Sciences, Xiamen University, Xiamen 361002, China
cDepartment of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
dDepartment of Materials Science and Engineering, College of Materials, Xiamen University, Xiamen 361005, China
First published on 9th February 2015
Abstract
Lipophilicity enhancement of mitomycin C (MMC) was achieved by the introduction of soybean phosphatidyhlcholine (SPC, a kind of phospholipid) (Molecular Pharmaceutics, 2013, 10, 90–101). In addition, the co-delivery of both drugs with one kind of nanoscale drug carrier provided a promising strategy to realize synergistic therapeutic effects and overcome drug resistance in cancer therapy. In this work, we developed folate (FA) functionalized MMC–SPC phospholipid complexes and 10-hydroxycamptothecin (HCPT)-loaded micelles (MMC/HCPT loaded FA-micelles) by film hydration followed by a dialysis and extrusion technique. The MMC/HCPT loaded FA-micelles possessed a nanoscale particle size, a well-controllable drug loading efficiency, and simultaneously sustained and pH-dependent drug release. In vitro cellular uptake analysis suggested that the MMC/HCPT loaded FA-micelles could be efficiently taken up by cancer cells via FA receptor-mediated endocytosis. In vitro cell viability studies demonstrated that the MMC/HCPT loaded FA-micelles showed time- and concentration-dependent cytotoxicity, and significantly enhanced the cytotoxicity compared to both free drugs. Moreover, the MMC/HCPT loaded FA-micelles can simultaneously deliver both MMC and HCPT to not only efficiently promote their accumulation in the tumor as a result of passive and active targeting, but also sufficiently inhibit the tumor growth compared to treatment with both free drugs while reducing the toxicity. The both MMC and HCPT anticancer drug-loaded FA-micelles can be considered as effective therapeutic systems for targeted drug co-delivery and combination cancer chemotherapy.
1. Introduction
Cancer remains one of the most devastating diseases in the world, which highlights the urgent need for alternative therapeutic strategies.1 Current combination cancer chemotherapy with two or more anticancer drugs has attracted increasing attention to achieve synergistic therapeutic effects by either countering biological compensation or accessing context-specific multiple-target mechanisms.2–4 Mitomycin C (MMC) is a potent antibiotic and a promising hydrophilic anticancer drug with a broad spectrum of anticancer activity.5,6 Its anticancer mechanism is associated with DNA cross-linking, formation of monoadducts with DNA, and free radical-induced DNA strand breaks.7,8 MMC is a cell cycle phase-nonspecific drug, and an aziridine ring and a carbamoyl chain are essential for its pharmacological activity.9,10 MMC is also a very poor substrate for P-glycoprotein and retains activity against many types of P-glycoprotein-mediated multidrug resistant tumor cells.11–13 10-Hydroxycamptothecin (HCPT), along with its camptothecin analogs (CPTs: irinotecan, topotecan, etc.), is also a potent topoisomerase inhibitor and a promising hydrophobic anticancer drug with a broad spectrum of anticancer activity.14,15 Its anticancer mechanism has been associated with the formation of nuclear enzyme topoisomerase I–DNA cleavage complexes.16 HCPT is a typical S phase-specific drug, and a lactonic ring is essential for its pharmacological effect.15 It exists in two forms depending on the pH value, namely, an active lactone form at acidic pH and an inactive carboxylate form at neutral and basic pH.17 In previous studies, it was found that combination cancer therapy of MMC and CPT may be a useful alternative regimen for a variety of human cell carcinomas, including cervical cell carcinoma (also called as cervical squamous cell carcinoma), ovarian cell carcinoma, gastric cell carcinoma, etc.18–23 Nevertheless, some strategies are urgently needed to overcome the barriers which might hinder the simultaneous delivery of MMC and HCPT, for instance, the self-aggregation and precipitation of drugs in the bloodstream, the rapid elimination of drugs from the body, and the serious drug-related side effects.24–26 In addition, HCPT is difficult to use clinically owing to its poor solubility in water and reversible, pH-dependent instability between the active lactone and inactive carboxylate form.17One promising approach to overcome and achieve efficient cancer combination therapy is to introduce both the MMC and HCPT drugs into nanosized drug carriers. Nanosized drug carriers are emerging platforms for cancer therapy because of their unique ability to increase the water solubility and stability, improve the pharmacokinetic profile, prolong the circulation time, and promote the enrichment and accumulation of drugs in tumor tissue because of the enhanced permeability and retention (EPR) effect.27,28 Nanosized drug carriers-based combination cancer chemotherapy has the potential to reduce the toxicity and overcome the poorly controlled dosing of traditional systemic combination cancer chemotherapies.24,29,30 In addition, compared to delivering a single type of drug, simultaneously delivering multiple types of drugs with disparate physiochemical characteristics, distinct pharmaceutical pathways, and different anticancer mechanisms to the same tumor cells using a single kind of nanosized drug carrier could achieve additional or synergistic therapeutic effects by overcoming the drug resistance of cancer cells to one type of chemotherapeutic drug as well as reducing the serious side effects and limited regime of clinical use.3,24,31 However, it is still a challenge to effectively incorporate the water-soluble MMC drug into nanoscale drug carriers. As has been reported, water-soluble drug–phospholipid complexes, water-soluble drug–cyclodextrin complexes, and water-soluble drug–dextran complexes have received increasing attention owing to their remarkable improvements in the lipophilicity, effectiveness, and safety of the drug.32–36 The complexation of MMC with an amphipathic (hydrophilic and lipophilic) moiety such as soybean phosphatidylcholine (SPC, a kind of phospholipid) has also been developed to increase the bioavailability and efficacy of MMC, and more importantly, to promote the incorporation of MMC into nanoscale drug delivery systems with high encapsulation capacity and low nonspecific release for cancer therapy.10,37–39
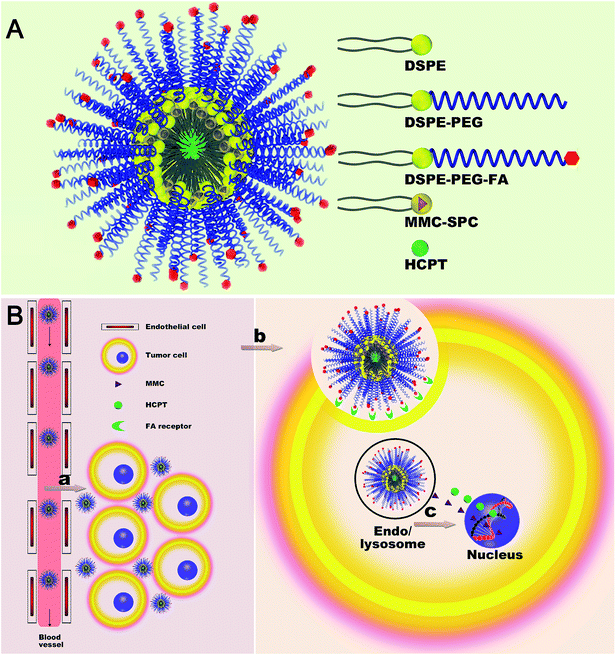
Recently, polymeric micelles with a nanoparticle structure, self-assembled from biocompatible and biodegradable amphiphilic block polymers, have attracted increasing attention as drug carriers for anticancer drugs.40 Hydrophobic blocks form the inner core of the micelles, and hydrophilic blocks form the outer shell. Among different amphiphilic block copolymers, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)] (DSPE-PEG) has attracted much attention, as it is an FDA-approved pharmaceutical excipient.41,42 Moreover, DSPE-PEG easily forms micelles with a small particle size and a low critical micelle concentration (CMC) (∼5 μM) due to the combination of a hydrophilic PEG chain and extremely hydrophobic long chain fatty acids.43,44 As the folate (FA) receptors are overexpressed on a wide variety of cancer cells,45 FA was used as a targeting ligand to functionalize the surface of DSPE-PEG-based micelles, offering cancer-specific targeting of drug carriers for the efficient co-delivery of MMC/HCPT to the tumor while minimizing the side effects.
In this work, we explored the feasibility of employing DSPE, DSPE-PEG, and DSPE-PEG-FA mixed micelles as a co-delivery system for both MMC and HCPT (Scheme 1A). Both MMC and HCPT loaded DSPE, DSPE-PEG, and DSPE-PEG-FA mixed micelles (MMC/HCPT loaded FA-micelles) were designed with a core–shell architecture: an inner reservoir core consisting of hydrophobic DSPE to encapsulate both MMC and HCPT drugs; an outer protective shell consisting of hydrophilic PEG to extend the circulation time; and a tumor-targeting ligand FA on the surface to increase the selectivity. Due to the nanoscale structure, the MMC/HCPT loaded FA-micelles could passively deliver and accumulate themselves at the tumor site, possibly via the EPR effect. After the selective cellular internalization of the MMC/HCPT loaded FA-micelles via FA receptor-mediated endocytosis, both MMC and HCPT drugs would be co-released to induce the death of tumor cells (Scheme 1B).
2. Materials and methods
2.1. Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and cell culture media were from Invitrogen (Carlsbad, CA, USA) unless otherwise noted. Mitomycin C (MMC) was purchased from Hisun Pharmaceutical Co. Ltd (Zhengjiang, China). 10-Hydroxycamptothecin (HCPT) was provided by Huangshi Pharmaceutical Co. Ltd (China). Soybean phosphatidylcholine (SPC) was provided by Lipoid GmbH (Germany). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine (DSPE), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy(polyethyleneglycol)-2000 (DSPE-PEG), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-folate(polyethyleneglycol)-2000 (DSPE-PEG-FA) were purchased from Avanti Polar Lipids (USA). Folate (FA) was purchased from Bio Basic Inc. (Canada). Cy5.5 mono NHS ester (Cy5.5-NHS) was from Fanbo Biochemicals (China).2.2. Preparation of MMC/HCPT-loaded FA-micelles
The MMC/HCPT-loaded FA-micelles were prepared by a film dispersion method. Briefly, DSPE, DSPE-PEG, DSPE-PEG-FA, and MMC–SPC phospholipid complexes (prepared at the weight ratio of MMC and SPC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 following a published protocol)10 dissolved in chloroform, together with HCPT dissolved in chloroform/methanol (volume ratio of chloroform and methanol = 1
1 following a published protocol)10 dissolved in chloroform, together with HCPT dissolved in chloroform/methanol (volume ratio of chloroform and methanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were transferred to a round-bottom flask at a mass ratio of DSPE
1) were transferred to a round-bottom flask at a mass ratio of DSPE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPSE-PEG
DPSE-PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DSPE-PEG-FA
DSPE-PEG-FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MMC–SPC
MMC–SPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCPT = 1
HCPT = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.72
0.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.18. The solvent was removed by vacuum rotary evaporation to form a homogeneous thin drug-containing lipid film. The film was further dried under vacuum overnight to remove any residual solvent. The dried film was hydrated with HEPES-buffered saline (HBS, pH 7.4) followed by vigorous vortexing for 15 min at room temperature. The mixture was transferred into a dialysis tube (MWCO 3500) to be dialyzed against HBS. The micelle suspensions were filtered through 200 nm and 100 nm polycarbonate membranes. The MMC/HCPT-loaded micelles were prepared using the identical procedure except that DSPE-PEG-FA was replaced by DSPE-PEG at the equivalent amount for comparison in most of the experiments. In addition, the MMC and HCPT-loaded FA-micelles-Cy5.5 and MMC/HCPT-loaded micelles-Cy5.5 for in vivo imaging investigations were obtained by the same method except that the micelles were decorated with a dye-labeled lipid (this dye-labeled lipid was prepared using DSPE-PEG-NH2 and Cy5.5-NHS at a 1
0.18. The solvent was removed by vacuum rotary evaporation to form a homogeneous thin drug-containing lipid film. The film was further dried under vacuum overnight to remove any residual solvent. The dried film was hydrated with HEPES-buffered saline (HBS, pH 7.4) followed by vigorous vortexing for 15 min at room temperature. The mixture was transferred into a dialysis tube (MWCO 3500) to be dialyzed against HBS. The micelle suspensions were filtered through 200 nm and 100 nm polycarbonate membranes. The MMC/HCPT-loaded micelles were prepared using the identical procedure except that DSPE-PEG-FA was replaced by DSPE-PEG at the equivalent amount for comparison in most of the experiments. In addition, the MMC and HCPT-loaded FA-micelles-Cy5.5 and MMC/HCPT-loaded micelles-Cy5.5 for in vivo imaging investigations were obtained by the same method except that the micelles were decorated with a dye-labeled lipid (this dye-labeled lipid was prepared using DSPE-PEG-NH2 and Cy5.5-NHS at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in dimethyl sulfoxide at 4 °C for complete labeling of the lipid, and then lyophilized and stored as a powder at −20 °C), and then DSPE-PEG-Cy5.5 was incorporated into the micelles at about 0.5 mol% of total lipids to achieve an equivalent Cy5.5 concentration.
1 ratio in dimethyl sulfoxide at 4 °C for complete labeling of the lipid, and then lyophilized and stored as a powder at −20 °C), and then DSPE-PEG-Cy5.5 was incorporated into the micelles at about 0.5 mol% of total lipids to achieve an equivalent Cy5.5 concentration.
2.3. Characterization of MMC/HCPT-loaded FA-micelles
The lyophilized MMC/HCPT-loaded FA-micelles were studied using FTIR. The lyophilized MMC/HCPT-loaded FA-micelles were re-dispersed in PBS and studied by UV-vis analysis. These results are included in Fig. S1 in the ESI.†The average particle size, polydispersity index (PDI), and zeta potential of the MMC/HCPT-loaded FA-micelles were studied by dynamic light scattering (DLS) using a Malvern Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK). Particle size was evaluated by intensity distribution. The morphology of the MMC/HCPT-loaded FA-micelles was visualized by TEM (JEM 1400, JEOL, Tokyo, Japan). The amount of MMC in the MMC/HCPT-loaded FA-micelles was measured by HPLC (Waters Associates, Milford, MA, USA) as described in our previous reports.32,40 The amount of HCPT in the MMC/HCPT-loaded FA-micelles was determined by UV-vis measurement at 383 nm. The drug entrapment efficiency and drug loading content of MMC and HCPT were calculated according to the equation: drug encapsulation efficiency (%) = (the weight of the loaded drug/the weight of the input drug) × 100%. Drug loading content (%) = (the weight of the loaded drug/the weight of the drug-loaded micelles) × 100%.
2.4. In vitro drug release
To evaluate the release profile of MMC and HCPT from the MMC/HCPT-loaded FA-micelles, the release study was performed using a dialysis method. The MMC/HCPT-loaded FA-micelles (3 mg of MMC) were loaded into a dialysis bag (MWCO 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and immediately dialyzed against phosphate buffer solution (PBS) at 37 °C under constant stirring. At the predesigned time, the release medium was withdrawn and subsequently replaced with the same volume of fresh PBS. The release of MMC was determined by the HPLC method as described above. The release of HCPT was determined by using a fluorescence spectrophotometer (FLS920, Edinburgh Analytical Instruments, Edinburgh, UK) with an excitation wavelength of 382 nm using a standard calibration curve.46 The free MMC plus HCPT was used for comparison.
000) and immediately dialyzed against phosphate buffer solution (PBS) at 37 °C under constant stirring. At the predesigned time, the release medium was withdrawn and subsequently replaced with the same volume of fresh PBS. The release of MMC was determined by the HPLC method as described above. The release of HCPT was determined by using a fluorescence spectrophotometer (FLS920, Edinburgh Analytical Instruments, Edinburgh, UK) with an excitation wavelength of 382 nm using a standard calibration curve.46 The free MMC plus HCPT was used for comparison.
2.5. Cell culture
Human cervical carcinoma HeLa cells used as an FA receptor-overexpressing tumor cell model were cultured in FA-deficient Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. HeLa cells expressed a high level of FA receptor. All of the cells were cultivated in an incubator (Thermo Scientific) at 37 °C with 5% CO2.2.6. In vitro cellular uptake
Confocal laser scanning microscopy was used for qualitative observation of cellular uptake of the micelles. HeLa cells (5 × 105 per well) were seeded in 6-well plates at 37 °C and then incubated with the MMC/HCPT-loaded FA-micelles (0.005 mg mL−1 of MMC concentration) for 6 h. The cells were washed with PBS, fixed with 4% paraformaldehyde, stained with PI and imaged using a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems, Mannheim, Germany). HeLa cells treated with the MMC/HCPT-loaded micelles at the equivalent HCPT concentration were used for comparison.The cellular uptake of the micelles was further quantitatively analyzed by fluorescence measurement. HeLa cells (2 × 106 per well) were seeded into 6-well plates at 37 °C and then incubated with the MMC/HCPT-loaded FA-micelles. After removal of the culture medium, the cells were washed three times with cold PBS and then trypsinized and resuspended in PBS. The suspensions were centrifuged at 1000 rpm and 4 °C for 5 min. The supernatants were discarded and the cells were washed with PBS to remove the background fluorescence in the medium. After two cycles of washing and centrifugation, the cells were resuspended with PBS, disrupted by sonication, and analyzed by fluorescence measurement. HeLa cells treated with the MMC/HCPT-loaded micelles at the equivalent HCPT concentration were used for comparison.
2.7. In vitro cytotoxicity
The cytotoxicity of the MMC/HCPT-loaded FA-micelles was measured using an MTT assay according to the manufacturer’s suggested procedures. HeLa cells were exposed to the MMC/HCPT-loaded FA-micelles with different MMC and HCPT concentrations for 24 h. The data were expressed as the percentage of surviving cells. The MMC/HCPT-loaded micelles and free MMC plus HCPT were used for comparison.2.8. Animals and tumor models
All the animal procedures complied with the guidelines of the Xiamen University Institutional Animal Care and Use Committee. Adult Sprague-Dawley rats (200 ± 20 g) and BALB/C nude mice aged 5 weeks (16–20 g) were provided from Shanghai Laboratory Animal Center, Chinese Academy of Sciences. The tumor model was established by subcutaneous inoculation of 1 × 106 HeLa cells in selected positions in BALB/C nude mice. The greatest longitudinal diameter (length) and the greatest transverse diameter (width) of the tumor were measured using a caliper and the tumor volume was calculated using length × width2 × 0.5.2.9. In vivo pharmacokinetics
200 μL of the MMC/HCPT-loaded FA-micelles (3.2 mg MMC per kg and 1.6 mg HCPT per kg) was injected intravenously through the tail vein of rats. At timed intervals, 200 μL of blood was collected in a heparinized tube. The concentration of MMC and HCPT was quantified by the HPLC method and fluorescence spectrophotometry, respectively as described above. The pharmacokinetic parameters such as elimination half-life (t1/2), area under the plasma concentration versus the time curve (AUC), mean residence time (MRT), and total body clearance (CL) were calculated by fitting the blood drug pharmaceutical concentrations to a two-compartment model using WinNonlin software (version 5.1, Pharsight, Sunnyvale, CA). The MMC/HCPT-loaded micelles and free MMC plus HCPT at the equivalent HCPT concentration were used for comparison.2.10. In vivo fluorescence imaging
For in vivo fluorescence imaging, the MMC/HCPT-loaded FA-micelles-Cy5.5 were injected intravenously into female mice bearing HeLa tumors. Imaging was performed at 12 h after intravenous injection by a Maestro™ in vivo imaging system (Cambridge Research & Instrumentation, Woburn, MA, USA). Finally, the mice were sacrificed. The tumor and normal tissues (heart, lung, kidney, spleen, and liver) were excised, followed by washing the surface with 0.9% NaCl for the ex vivo fluorescence imaging using a Maestro™ in vivo imaging system. The resulting data can be used to identify, separate, and remove the contribution of auto fluorescence in the analyzed images. Mice treated with the MMC/HCPT-loaded micelles-Cy5.5 at the equivalent Cy5.5 concentration were used for comparison.2.11. In vivo anticancer effect
We used HeLa tumor bearing mice as a model to investigate the in vivo anticancer effect. When the tumor volume was about 50–100 mm3, the treatment was started. The tumor-bearing mice were randomly divided into 4 groups (6 mice per group) and were intravenously administrated with normal saline (the control group), MMC plus HCPT solution (3.2 mg MMC per kg and 1.6 mg HCPT per kg), MMC/HCPT-loaded micelles or MMC/HCPT-loaded FA-micelles at the equivalent MMC or HCPT concentration, respectively. The intravenous administration was continued three times at three day intervals through the tail vein. Each mouse was earmarked and followed individually throughout the experiments. The tumor volume and body weight were then monitored every two days for two weeks until the mice were euthanized.3. Results and discussion
3.1. Preparation of MMC/HCPT-loaded FA-micelles
The MMC/HCPT-loaded FA-micelles were fabricated with DSPE, DSPE-PEG, and DSPE-PEG-FA by the film hydration technique followed by the dialysis and extrusion method. DSPE, DSPE-PEG, and DSPE-PEG-FA via the self-assembly process formed a hydrophobic DSPE core–hydrophilic PEG shell structure with a FA moiety, and MMC–SPC and HCPT were encapsulated in the micelles. Specifically, SPC, DSPE, and DSPE-PEG served as the drug-loaded material, FA served as the targeting moiety, and both MMC and HCPT were used as the therapeutic agents for combination cancer chemotherapy. Combination cancer therapy with the simultaneous co-delivery of different pharmacologically therapeutic drugs to the same tumor cells could increase the anticancer efficacy because both therapeutic drugs can act via some pathways and the dosage of each individual therapeutic drug could be decreased, therefore reducing the toxic side effects against normal cells. The MMC and HCPT drug encapsulation efficiencies were 93.2 ± 1.7% and 91.8 ± 1.3%, respectively. The MMC and HCPT drug loading contents were 7.4 ± 0.6% and 3.6 ± 0.5%, respectively.3.2. Characterization of MMC/HCPT-loaded FA-micelles
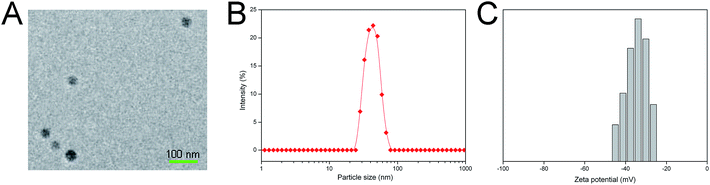
Table S1† shows the particle size and zeta potential of both the MMC/HCPT-loaded micelles and MMC/HCPT-loaded FA-micelles. Both the micellar formulations showed a hydrodynamic particle size of approximately 30–50 nm, which is typical for a micellar system composed of a mixture of lipid components. Both the micellar formulations also showed a net negative charge of −40 to −30 mV, due to the partial negative charge from the lipids on the surface of the micelles, which suggested that the micelles were stable in aqueous medium. In addition, both the micellar formulations were stable in aqueous solution at 37 °C for 5 days, as evidenced by the less than 10% decrease in the minimal change in the hydrodynamic particle size of both the micellar formulations (see Fig. 2, as discussed below). We also tested the fluorescence stability of the Cy 5.5 labeled micelles suspensions using a fluorescence spectrometer (F900, Edinburgh Instruments Ltd, UK). After one week of storage at 4 °C, the fluorescence intensity of the Cy 5.5 labeled micelles remained at 95.6 ± 1.2% of its initial intensity. The well-dispersed spherical morphology (Fig. 1A), nanoscale particle size (Fig. 1B), narrow particle size distribution, negative surface charge (Fig. 1C), high drug entrapment efficiency, and excellent physiological stability of the MMC/HCPT-loaded FA-micelles indicated that the MMC/HCPT-loaded FA-micelles were expected to be effective nanoscale drug delivery systems for intravenous administration.283.3. In vitro stability
The change in the hydrodynamic particle size of the micelles is a simple but essential index to estimate the stability of the micelles under various physiological conditions. An in vitro stability study of the MMC/HCPT-loaded FA-micelles was performed. We performed an in vitro stability study of the MMC/HCPT-loaded FA-micelles under various conditions: H2O, PBS, and 10% (v/v) plasma/heparin in PBS for 5 days at 37 °C (Fig. 2). No significant change in the hydrodynamic particle size of the MMC/HCPT-loaded FA-micelles (0.005 mg mL−1 of MMC concentration) was observed in different physiological environments even after 5 days, indicating the effective physiological stability of the micelles against ionic strength and protein adsorption. These results could be due to the electrostatic repulsion, steric stabilization, and structural integrity of the MMC/HCPT-loaded FA-micelles.3.4. In vitro drug release
The ideal drug delivery system for cancer therapy should retain the drug during blood circulation with minimal drug loss while releasing the drug in the tumor tissues.17 In vitro release profiles of free MMC plus HCPT, MMC/HCPT-loaded micelles, and MMC/HCPT-loaded FA-micelles were obtained in PBS at 37 °C at pH 7.4. No significant differences in the release rate of both MMC and HCPT were observed between the MMC/HCPT-loaded micelles and MMC/HCPT-loaded FA-micelles (Fig. 3A and B). The result implied that the modification with FA had no significant influence on the drug release behavior of the drug-loaded micelles (the MMC–SPC complex had suffered dissociation and MMC could be easily released in a free form).In vitro release profiles of MMC and HCPT from the MMC/HCPT-loaded FA-micelles were obtained in phosphate or acetate buffer at different pH values in order to imitate different conditions in vivo. It was reported that the pH values in blood circulation, tumor interstitium, and endo/lysosomes are about 7.4, 6.5, and 5.5, respectively.47 As shown in Fig. 3C, increased release of MMC from the MMC/HCPT-loaded FA-micelles was clearly observed as the pH decreased from 7.4 via 6.5 to 5.5. The result indicated the micelles were relatively stable under normal physiological conditions with low nonspecific drug release.48 Nevertheless, MMC was released more rapidly from the MMC/HCPT-loaded FA-micelles in the endo/lysosomes within the tumor cells, which contributed to enhancing the anticancer activity. However, as shown in Fig. 3D, the decreased release of HCPT from the MMC/HCPT-loaded FA-micelles was observed as the pH decreased from 7.4 to 6.5. This could be due to the conversion of the soluble carboxylate form into the insoluble lactone form in the low pH environment, and thus the solubility of the HCPT was greatly decreased.17 But the release of HCPT still displayed a controlled drug release behavior at pH 7.4, which indicated the micelles could maintain and preserve HCPT to some extent.17
3.5. In vitro cellular uptake
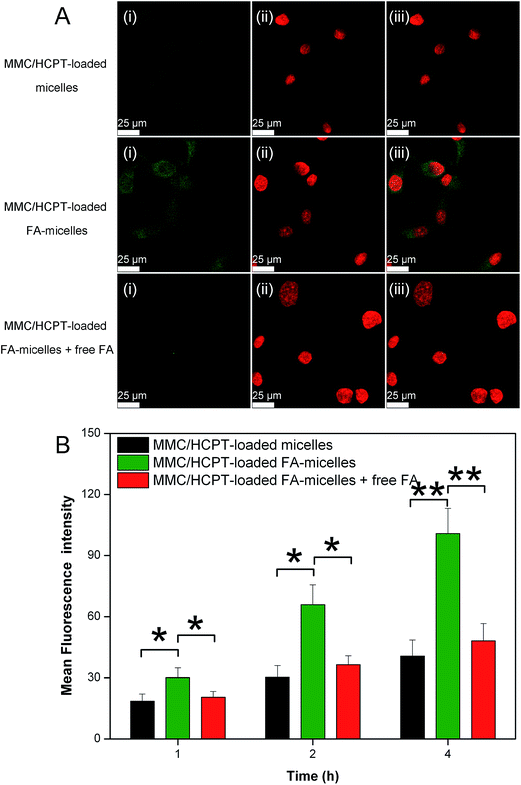
Human cervical cancer HeLa cells are well-known to overexpress FA receptors, which have a specific, high FA-binding affinity. To confirm the presence of FA on the micelles’ surface, we used HeLa cells as a model to investigate the interaction between the micelles and cancer cells by confocal laser scanning microscopy and fluorescence measurement.As observed in Fig. 4A, stronger green fluorescence was observed in HeLa cells treated with the MMC/HCPT-loaded FA-micelles compared to the MMC/HCPT-loaded micelles after the same incubation time. This result indicated that the presence of FA on the micelles’ surface greatly promoted the cellular uptake of the micelles by the targeted cells via FA receptor-mediated endocytosis. To validate the specificity of the cellular uptake of the MMC/HCPT-loaded FA-micelles, the FA competition experiment was carried out. The cellular uptake of the MMC/HCPT-loaded FA-micelles by excess free FA-pretreated HeLa cells was effectively blocked compared with the unpretreated HeLa cells. These results demonstrated that the high affinity of FA towards FA receptor-overexpressing cancer cells can effectively result in high cellular uptake efficiency.
Also, as shown in Fig. 4B, the mean fluorescence intensity for HeLa cells incubated with the MMC/HCPT-loaded FA-micelles was higher than that for HeLa cells incubated with the MMC/HCPT-loaded micelles after incubation for 1, 2, and 4 h. In the meantime, with the competitive binding effect of free FA, the mean fluorescence intensity decreased. The results from both confocal laser scanning microscopy and fluorescence measurement proved that the FA receptor mediated endocytosis significantly enhanced the cellular internalization efficiency of the MMC/HCPT-loaded FA-micelles and thus increased the accumulation of both drugs in targeted cells.
3.6. In vitro cytotoxicity
Motivated by the above result that more HCPT molecules in nuclei were detected in the cells after incubation with the MMC/HCPT-loaded FA-micelles compared to the MMC/HCPT-loaded micelles, the cytotoxicity of the MMC/HCPT-loaded FA-micelles was investigated in the HeLa cell line by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. It was found that the cell viability was not altered significantly by the drug-free micelles themselves (made of SPC, DSPE, DSPE-PEG, and DSPE-PEG-FA), suggesting that these materials are biocompatible for clinical applications (Fig. 5A and B). In addition, both the MMC/HCPT-loaded micelles and MMC/HCPT-loaded FA-micelles exhibited dose- and time-dependent cytotoxicity as expected. Moreover, the MMC/HCPT-loaded FA-micelles showed a significantly higher cytotoxicity in comparison to the MMC/HCPT-loaded micelles. The result indicated that FA could greatly facilitate the uptake of MMC/HCPT-loaded FA-micelles by HeLa cells and elevate the intracellular drug concentration of both MMC and HCPT.In addition, delivering both drugs by the MMC/HCPT-loaded FA-micelles with the dose- and time-dependent cytotoxicity significantly decreased the cell viability compared with delivering both drugs by the free MMC plus HCPT (Fig. 5A and B). In other words, the free MMC plus HCPT and MMC/HCPT-loaded micelles needed higher dosages to reach the same efficacy compared with the MMC/HCPT-loaded FA-micelles. These results revealed that the encapsulation of MMC and HCPT in the micelles and the specific FA recognition exerted an important role in the enhancement of cellular uptake and thus cytotoxic activity. In addition, the free MMC plus HCPT could cause the side effects due to the nonspecific drug release and nonselective cellular uptake, whereas the sustained drug release and active targeting ability of the MMC/HCPT-loaded FA-micelles had great potential to increase the therapeutic efficacy to tumor cells and reduce the side effects to normal cells in vivo.
More notably, to prove the synergistic effect of the MMC/HCPT-loaded FA-micelles, we used the Chou-Talalay method to calculate the combination index (CI).49 CI values lower than, equal to, and higher than 1 represented synergism, additive, and antagonism, respectively (synergism < 1, additive = 1 and antagonism > 1).49,50 The CI of the MMC/HCPT-loaded micelles was calculated as 0.37 ± 0.05, and more importantly, the CI of the MMC/HCPT-loaded FA-micelles was calculated as 0.22 ± 0.04. These results indicated the synergistic actions of both MMC and HCPT. Despite an active targeting effect, the highly synergistic effect possibly resulted from the synergies between the distinct modes of action and different anticancer mechanisms of both MMC and HCPT.
3.7. In vivo pharmacokinetics
To evaluate the therapeutic potential of the MMC/HCPT-loaded FA-micelles, we examined the in vivo pharmacokinetics of the MMC/HCPT-loaded FA-micelles.The plasma concentration–time curves of MMC following intravenous injection of the free MMC plus HCPT, MMC/HCPT-loaded micelles, and MMC/HCPT-loaded FA-micelles are shown in Fig. 6A, and the pharmacokinetic parameters are summarized in Table S2 in the ESI.† The plasma concentration of MMC in rats intravenously injected with the free MMC plus HCPT significantly decreased over time, indicating MMC was rapidly cleared from the bloodstream after intravenous injection. However, the MMC/HCPT-loaded micelles and MMC/HCPT-loaded FA-micelles significantly improved the pharmacokinetic parameters of MMC in comparison with the free MMC plus HCPT, as indicated by the significantly higher area under the plasma concentration versus time curves (AUC), longer elimination half-life (t1/2β), longer mean residence time (MRT), and lower total body clearance (CL) (Table S2†). Specifically, the AUC0–∞, t1/2β, and MRT0–∞ for the MMC/HCPT-loaded FA-micelles were 13.2-, 12.3-, and 19.3-fold higher than those of the free MMC plus HCPT, respectively, while the CL for the MMC/HCPT-loaded micelles was 8.2-fold lower than that of the free MMC plus HCPT, implying an enhanced blood persistence of MMC in the circulation system.
The plasma concentration–time curves of HCPT following intravenous injection of the free MMC plus HCPT, MMC/HCPT-loaded micelles, and MMC/HCPT-loaded FA-micelles are shown in Fig. 6B, and the pharmacokinetic parameters are summarized in Table S3.† The plasma concentration of HCPT in rats intravenously injected with the free MMC plus HCPT also significantly decreased over time. However, the MMC/HCPT-loaded FA-micelles significantly slowed the decrease of the plasma concentration of HCPT, showing a higher t1/2β with a higher AUC0–∞. The enhanced blood circulation time of HCPT caused by the MMC/HCPT-loaded FA-micelles was also evidenced by the increase of MRT0–∞ and the decrease of CL in comparison with the free MMC plus HCPT. The reduced clearance rate of HCPT in the circulation system was probably due to their high colloidal stability and low protein absorption. Based on the above results, we inferred that the prolonged circulation time and controlled drug release of the MMC/HCPT-loaded FA-micelles would offer effective transport of the micelles across tumor vasculature and thus favor a dual active-plus-passive targeting mechanism.
3.8. In vivo fluorescence imaging
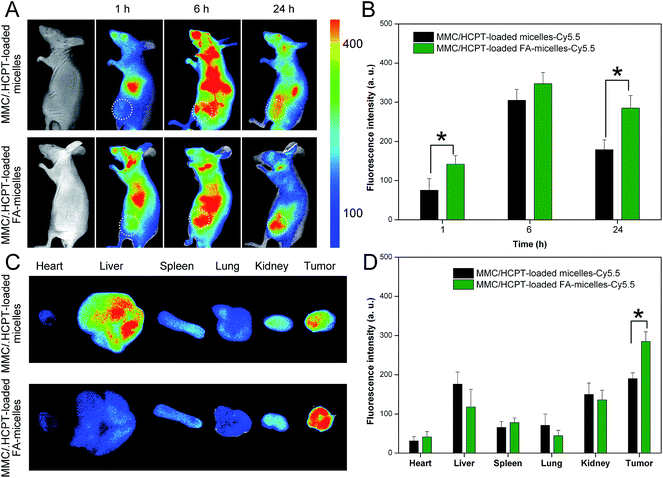
A noninvasive real-time imaging approach was used to investigate the tumor targeting and in vivo biodistribution of the Cy5.5-labeled MMC/HCPT loaded FA-micelles in mice from 1 to 24 h post injection. The in vivo fluorescence images and the corresponding tumor fluorescence intensities of the mice treated with the MMC/HCPT loaded micelles-Cy5.5 and the MMC/HCPT loaded FA-micelles-Cy5.5 are shown in Fig. 7A and B, respectively. The mice treated with the MMC/HCPT loaded micelles-Cy5.5 and MMC/HCPT loaded FA-micelles-Cy5.5 clearly exhibited accumulation in the tumor, which could be ascribed to the EPR effect mediated passive tumor targeting (Fig. 7A). In addition, the mice treated with the MMC/HCPT loaded micelles-Cy5.5 and MMC/HCPT loaded FA-micelles-Cy5.5 showed a similar change of the fluorescence intensity as a function of time in the tumor (Fig. 7B). It was noteworthy that the mice treated with the MMC/HCPT loaded FA-micelles-Cy5.5 at 24 h post injection still retained significantly higher fluorescence intensity in the tumor compared with the mice treated with the MMC/HCPT loaded micelles-Cy5.5 (Fig. 7B). The result could be ascribed to the passive plus active tumor targeting effect (see Fig. 4) and long blood circulation time (see Fig. 6). The rapid and effective cellular uptake and internalization of nanoscale drug delivery systems in vivo would create a great diffusion gradient which would encourage and boost the directed transport of nanoscale drug delivery systems into the tumor tissues, and thus improve the efficiency of tumor-targeted drug delivery.The tumor and major organ distribution of the mice treated with the MMC/HCPT loaded micelles-Cy5.5 and MMC/HCPT loaded FA-micelles-Cy5.5 after 24 h post injection is also shown in Fig. 7C and D. The fluorescence signals of the mice treated with the MMC/HCPT loaded micelles-Cy5.5 were mainly located in the tumor, liver, and kidney. However, the MMC/HCPT loaded FA-micelles-Cy5.5 obviously promoted the accumulation of the fluorescence signals in the tumor. These results provided further evidence for the tumor-specific targeting effect, proving that the MMC/HCPT loaded FA-micelles are suitable to achieve long-term in vivo retention and superior tumor targeting.
3.9. In vivo anticancer effect
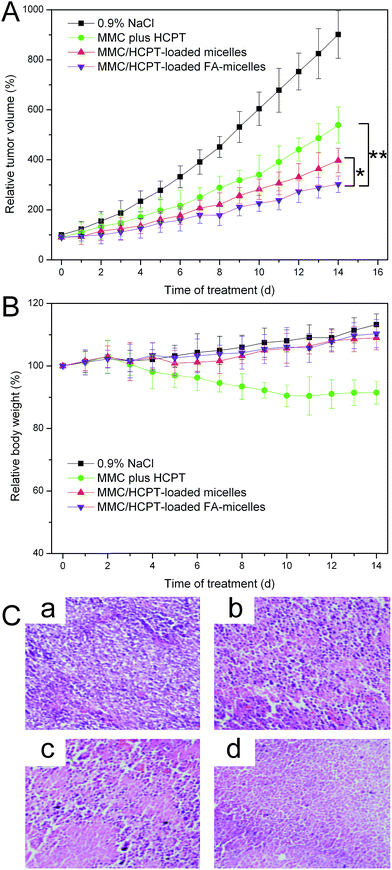
The MMC/HCPT loaded FA-micelles were anticipated to show excellent anticancer efficiency and low side effects because of their high accumulation in the tumour, long blood circulation, and good biocompatibility. Thus, the in vivo anticancer effect was investigated to provide more direct evidence for the anticancer potential of the MMC/HCPT loaded FA-micelles. As observed in Fig. 8A, compared to the control group, the relative tumor volumes of the free MMC plus HCPT, MMC/HCPT loaded micelles, and MMC/HCPT loaded FA-micelles groups were significantly decreased after a schedule of multiple doses, implying a significant inhibition of tumor growth. It was noted that the MMC/HCPT loaded FA-micelles groups produced the most inhibition of tumor growth. The nanoscale characteristics, PEGylation, and FA functionalization most likely allowed the MMC/HCPT loaded FA-micelles to achieve a dual active-plus-passive targeting strategy (preferential accumulation at the tumor site by the EPR effect and sufficient internalization within the tumor cells by FA receptor-mediated endocytosis), and thus significantly enhanced the anticancer efficacy of the MMC/HCPT loaded FA-micelles.37 In addition, in our previous work, the preparation of a water soluble drug–phospholipid complex (MMC–SPC phospholipid complex) improved the lipophilicity and liposolubility, increasing the MMC drug encapsulation efficacy and protecting the MMC drug against degradation in vitro and in vivo.10 Moreover, the lipophilicity of the MMC-loaded nanoscale drug delivery system in the tumor tissues also facilitated the drug delivery to the interior of the tumor cells across the phospholipid bilayer of cellular membranes.Additional evidence of the improved anticancer efficacy of the MMC/HCPT loaded FA-micelles was obtained by observing the H&E stained images (Fig. 8C). Compared to the control group, some obvious cell death was observed in the free MMC plus HCPT and MMC/HCPT loaded micelles group. In addition, the MMC/HCPT loaded FA-micelles group showed significant severe cell death, further confirming the superior anticancer effect. These results suggested that the cancer therapy of the MMC/HCPT loaded FA-micelles was more efficient in reducing the cell division and proliferation, and inducing the cell apoptosis and necrosis compared to the MMC/HCPT loaded micelles and the free drugs, which was attributed to the extended blood circulation time, the EPR effect mediated tumor accumulation, and FA receptor-mediated cellular uptake. More importantly, the mixed micelles, as ideal drug co-delivery systems, could synchronously and specifically deliver both MMC and HCPT into the tumor site and thus release both MMC and HCPT in a controlled and sustained manner, which would be conducive to the more effective synergistic or additive anticancer efficiency.
The potential toxicity presents one of the major obstacles for cancer chemotherapy, especially during chronic administration.24 In this study, the potential toxicity of the MMC/HCPT loaded FA-micelles was investigated by monitoring the change of animal behavior and body weight. The mice appeared lively throughout the study, and no side effects such as obvious body weight loss or abnormal animal behavior were observed in the MMC/HCPT loaded FA-micelles and MMC/HCPT loaded micelles group (Fig. 8B). On the contrary, compared to the control group, the free MMC plus HCPT group showed observable body weight loss with some clinical symptoms, such as reduced feeding, depression, and listlessness. All of these results indicated that the MMC/HCPT loaded FA-micelles showed excellent therapeutic effects while reducing the toxicity which would greatly improve the quality of life of the patient.
4. Conclusions
In this study, well-defined MMC/HCPT loaded FA-micelles were successfully prepared using the film hydration technique followed by the dialysis and extrusion method for tumor-targeted co-delivery of both drugs. The MMC/HCPT loaded FA-micelles exhibited a small particle size, a narrow particle size distribution, and a controlled and sustained drug release. Based on an active-plus-passive targeting strategy via the EPR effect and FA receptor-mediated endocytosis, the MMC/HCPT loaded FA-micelles could be sufficiently internalized by tumor cells, deliver both drugs to the nucleus and finally aid the targeting to the tumor site. Cancer therapy using the MMC/HCPT loaded FA-micelles not only highly induced the death of tumor cells in vitro, but also sufficiently inhibited the growth of tumor tissue in vivo. Moreover, the MMC/HCPT loaded FA-micelles demonstrated reduced toxicity. Therefore, the MMC/HCPT loaded FA-micelles can be regarded as effective targeted co-delivery systems and have great potential for targeted combination cancer chemotherapy.Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant no. 31271071 and 81472458).References
- A. Jemal, F. Bray, M. M. Center, J. Ferlay, E. Ward and D. Forman, Ca-Cancer J. Clin., 2011, 61, 69–90 CrossRef PubMed.
- J. Woodcock, J. P. Griffin and R. E. Behrman, N. Engl. J. Med., 2011, 364, 985–987 CrossRef CAS PubMed.
- C. Walsh, Nature, 2000, 406, 775–781 CrossRef CAS PubMed.
- S. Jain, R. Jain, M. Das, A. K. Agrawal, K. Thanki and V. Kushwah, RSC Adv., 2014, 4, 29193–29201 RSC.
- S. T. Crooke and W. T. Bradner, Cancer Treat. Rev., 1976, 3, 121–139 CrossRef CAS.
- T. Sen, S. J. Sheppard, T. Mercer, M. Eizadi-sharifabad, M. Mahmoudi and A. Elhissi, RSC Adv., 2012, 2, 5221–5228 RSC.
- J. M. Silva, S. Khan and P. J. O’Brien, Biochem. Pharmacol., 1993, 45, 2303–2309 CrossRef CAS.
- M. Tomasz, R. Lipman, D. Chowdary, J. Pawlak, G. L. Verdine and K. Nakanishi, Science, 1987, 235, 1204–1208 CAS.
- S. T. Crooke and W. T. Bradner, Cancer Treat. Rev., 1976, 3, 121–139 CrossRef CAS.
- Z. Hou, Y. Li, Y. Huang, C. Zhou, J. Lin, Y. Wang, F. Cui, S. Zhou, M. Jia, S. Ye and Q. Zhang, Mol. Pharmaceutics, 2013, 10, 90–101 CrossRef CAS PubMed.
- R. Maitra, P. A. Halpin, K. H. Karlson, R. L. Page, D. Y. Paik, M. O. Leavitt, B. D. Moyer, B. A. Stanton and J. W. Hamilton, Biochem. J., 2001, 355, 617–624 CAS.
- S. Ekins, R. B. Kim, B. F. Leake, A. H. Dantzig, E. G. Schuetz, L.-B. Lan, K. Yasuda, R. L. Shepard, M. A. Winter, J. D. Schuetz, J. H. Wikel and S. A. Wrighton, Mol. Pharmacol., 2002, 61, 964–973 CrossRef CAS.
- A. A. Gabizon, D. Tzemach, A. T. Horowitz, H. Shmeeda, J. Yeh and S. Zalipsky, Clin. Cancer Res., 2006, 12, 1913–1920 CrossRef CAS PubMed.
- M. E. Wall, M. C. Wani, C. E. Cook, K. H. Palmer, A. T. McPhail and G. A. Sim, J. Am. Chem. Soc., 1966, 88, 3888–3890 CrossRef CAS.
- L. P. Rivory and J. Robert, Pharmacol. Ther., 1995, 68, 269–296 CrossRef CAS.
- A. Hatefi and B. Amsden, Pharm. Res., 2002, 19, 1389–1399 CrossRef CAS.
- Y. Zhang, C. Xiao, M. Li, J. Chen, J. Ding, C. He, X. Zhuang and X. Chen, Macromol. Biosci., 2013, 13, 584–594 CrossRef CAS PubMed.
- Y. Kano, K. Suzuki, M. Akutsu, K. Suda, Y. Inoue, M. Yoshida, S. Sakamoto and Y. Miura, Int. J. Cancer, 1992, 50, 604–610 CrossRef CAS.
- M. B. Lustberg, T. Bekaii-Saab, D. Young, G. Otterson, W. Burak, A. Abbas, B. McCracken-Bussa, M. E. Lustberg and M. A. Villalona-Calero, J. Thorac. Oncol., 2010, 5, 713–718 Search PubMed.
- K. Nishino, Y. Aoki, T. Amikura, H. Obata, M. Sekine, T. Yahata, K. Fujita and K. Tanaka, Gynecol. Oncol., 2005, 97, 893–897 CrossRef CAS PubMed.
- N. Umesaki, T. Fujii, R. Nishimura, T. Tanaka, M. Nishida, H. Fushiki, K. Takizawa, K. Yamamoto, K. Hasegawa, R. Izumi and G. Japan Gynecologic Oncology, Gynecol. Oncol., 2004, 95, 127–132 CrossRef CAS PubMed.
- T. Yamao, K. Shirao, Y. Matsumura, K. Muro, Y. Yamada, M. Goto, K. Chin and Y. Shimada, Ann. Oncol., 2001, 12, 1729–1735 CrossRef CAS.
- K. Kokawa, R. Nishimura, T. Fujii and N. Umesaki, Anticancer Res., 2007, 27, 2721–2727 CAS.
- J. Yao, L. Zhang, J. Zhou, H. Liu and Q. Zhang, Mol. Pharmaceutics, 2013, 10, 1080–1091 CrossRef CAS PubMed.
- J. den Hartigh, J. G. McVie, W. J. van Oort and H. M. Pinedo, Cancer Res., 1983, 43, 5017–5021 CAS.
- J. F. Pizzolato and L. B. Saltz, Lancet, 2003, 361, 2235–2242 CrossRef CAS.
- E. J. Cho, H. Holback, K. C. Liu, S. A. Abouelmagd, J. Park and Y. Yeo, Mol. Pharmaceutics, 2013, 10, 2093–2110 CrossRef CAS PubMed.
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- Y. Yan, M. Bjornmalm and F. Caruso, ACS Nano, 2013, 7, 9512–9517 CrossRef CAS PubMed.
- Y. Chen, H. Chen and J. Shi, Mol. Pharmaceutics, 2014, 11, 2495–2510 CrossRef CAS PubMed.
- F. Kratz and A. Warnecke, J. Controlled Release, 2012, 164, 221–235 CrossRef CAS PubMed.
- F. Cui, K. Shi, L. Zhang, A. Tao and Y. Kawashima, J. Controlled Release, 2006, 114, 242–250 CrossRef CAS PubMed.
- J. Khan, A. Alexander, Ajazuddin, S. Saraf and S. Saraf, J. Controlled Release, 2013, 168, 50–60 CrossRef CAS PubMed.
- G. Ling, P. Zhang, W. Zhang, J. Sun, X. Meng, Y. Qin, Y. Deng and Z. He, J. Controlled Release, 2010, 148, 241–248 CrossRef CAS PubMed.
- S. Sajeesh, K. Bouchemal, V. Marsaud, C. Vauthier and C. P. Sharma, J. Controlled Release, 2010, 147, 377–384 CrossRef CAS PubMed.
- C. Wu, M. Zhang, Z. Zhang, K. W. Wan, W. Ahmed, D. A. Phoenix, A. M. Elhissi and X. Sun, Mol. Pharmaceutics, 2014, 11, 3371–3377 CrossRef CAS PubMed.
- Y. Li, J. Lin, H. Wu, M. Jia, C. Yuan, Y. Chang, Z. Hou and L. Dai, J. Mater. Chem. B, 2014, 2, 6534–6548 RSC.
- Y. Li, H. Wu, M. Jia, F. Cui, J. Lin, X. Yang, Y. Wang, L. Dai and Z. Hou, Mol. Pharmaceutics, 2014, 11, 3017–3026 CrossRef CAS PubMed.
- Y. Li, H. Wu, X. Yang, M. Jia, Y. Li, Y. Huang, J. Lin, S. Wu and Z. Hou, Mol. Pharmaceutics, 2014, 11, 2915–2927 CrossRef CAS PubMed.
- A. S. Mikhail and C. Allen, J. Controlled Release, 2009, 138, 214–223 CrossRef CAS PubMed.
- T. Wei, J. Liu, H. Ma, Q. Cheng, Y. Huang, J. Zhao, S. Huo, X. Xue, Z. Liang and X. J. Liang, Nano Lett., 2013, 13, 2528–2534 CrossRef CAS PubMed.
- R. C. Huxford-Phillips, S. R. Russell, D. Liu and W. Lin, RSC Adv., 2013, 3, 14438–14443 RSC.
- V. P. Torchilin, Adv. Drug Delivery Rev., 2002, 54, 235–252 CrossRef CAS.
- S. Tong, S. Hou, B. Ren, Z. Zheng and G. Bao, Nano Lett., 2011, 11, 3720–3726 CrossRef CAS PubMed.
- P. S. Low, W. A. Henne and D. D. Doorneweerd, Acc. Chem. Res., 2008, 41, 120–129 CrossRef CAS PubMed.
- W. Li, X. Zhang, X. Hao, J. Jie, B. Tian and X. Zhang, Chem. Commun., 2013, 49, 10989–10991 RSC.
- E. Jin, B. Zhang, X. Sun, Z. Zhou, X. Ma, Q. Sun, J. Tang, Y. Shen, E. Van Kirk, W. J. Murdoch and M. Radosz, J. Am. Chem. Soc., 2013, 135, 933–940 CrossRef CAS PubMed.
- W. Zhang, J. Sun, Y. Liu, M. Tao, X. Ai, X. Su, C. Cai, Y. Tang, Z. Feng, X. Yan, G. Chen and Z. He, Mol. Pharmaceutics, 2014, 11, 3279–3290 CrossRef CAS PubMed.
- T. C. Chou and P. Talalay, Adv. Enzyme Regul., 1984, 22, 27–55 CrossRef CAS.
- S. Barua and S. Mitragotri, ACS Nano, 2013, 7, 9558–9570 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14602f |
| This journal is © The Royal Society of Chemistry 2015 |