Physicochemical performance of FeCO3 films influenced by anions
Chengqiang
Ren
*ab,
Wanguo
Wang
b,
Xing
Jin
b,
Li
Liu
b and
Taihe
Shi
a
aState Key Laboratory of Oil and Gas Reservoir Geology and Exploitation, Southwest Petroleum University, Chengdu, 610500, P. R. China. E-mail: chengqiangren@163.com; Fax: +86 28 83037406; Tel: +86 28 83037406
bSchool of Materials Science and Engineering, Southwest Petroleum University, Chengdu, 610500, P. R. China
First published on 2nd February 2015
Abstract
The corrosion film plays an important role in the further electrochemical processes of steel in CO2 corrosion. Thus, the physicochemical performance of an FeCO3 film was investigated using the Mott–Schottky electrochemical technique, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), X-ray diffraction (XRD) and scanning electron microscopy (SEM). The performance of the FeCO3 film was dependent on the aqueous solution. In 0.5 mol L−1 NaHCO3 solution, an n-type semiconducting behavior was found for the FeCO3 film. The dense microstructure and lower interstitial cation or anion vacancy doping was in favor of strong corrosion resistance of the film. On the contrary, a p-type semiconducting behavior of the FeCO3 film was exhibited in both 0.5 mol L−1 NaCl solution and 0.5 mol L−1 Na2SO4 solution. Higher cation vacancy doping was found, and the integrity of the microstructure was damaged, which decreased the transfer resistance of electron and mass. As a result, the protective ability of the FeCO3 film was decreased. The physicochemical mechanism for the semiconducting properties of the FeCO3 film was explained using the Point Defect Model (PDM).
1. Introduction
CO2 corrosion of steel has been one of the most important problems in the petroleum industry and CO2 capture process,1,2 which has caused thinning, perforation or fracture of tubular goods.Though many different electrochemical mechanisms have been proposed to explain the CO2 corrosion of steel, the overall reaction is presented as eqn (1):
| Fe + CO2 + H2O → FeCO3 + H2 | (1) |
The protective ability strongly depends on the properties of the FeCO3 film on the surface of the steel. The first requirement for corrosion inhibition is the integrity of the corrosion film. Thus, the mechanical performances of the corrosion film, including fracture toughness,7 wear resistance,8 Young’s modulus and adhesion strength,9 have been investigated, and their relationships to the protective behavior were also discussed.
The electrochemical behavior of the corrosion film is another important factor in anti-corrosion. The electrochemical characteristics were monitored during corrosion film formation. They indicated that the CO2 corrosion film promoted electrode potential and mass transferring resistance.10,11 The passive film on the iron base alloy presented semiconductor properties; for example, an n-type semiconductor formed on the surface of X70 steel in carbonate/bicarbonate solution.12 A deep understanding of the physicochemical process of the film in electrochemical corrosion can be realized from the semiconducting properties.
Literature has shown that there is a correlation between the corrosion behavior of a metal and the semiconducting properties of a passive film,13–15 but in the CO2 corrosion field, reports are scarcely found. Mott–Schottky measurement is a powerful method for studying semiconductor characteristics. The well known equations are listed as eqn (2) for an n-type semiconductor and eqn (3) for a p-type semiconductor, according to the Mott–Schottky theory:
 | (2) |
 | (3) |
As our previous work reported, the corrosion of an oil tube was severely affected by groundwater chemistry.17 Generally, anions play an important role in the corrosion. In Nesic’s review, the species of anions in the formation solution are Cl−, HCO3− and SO42−. For these reasons, the purpose of our work is to clarify the influence of Cl−, HCO3− and SO42− on the semiconducting properties of a FeCO3 film in order to understand the different corrosion behaviors of tubular steel in CO2 storage oil and gas wells.
2. Experimental
Material
The experimental material is N80 steel with the chemical composition (wt%) 0.24 C, 0.22 Si, 1.19 Mn, 0.013 P, 0.004 S, 0.036 Cr, 0.021 Mo, 0.028 Ni, and 98.248 Fe. The sample, a plate with a 10 × 10 mm2 surface and 3 mm thickness, was machined from the oil tube directly. The pretreatment of the sample included polishing with 800-grit silicon carbide paper, degreasing with acetone, washing with distilled water and drying with a blow drier, sequentially.Film preparation
Distilled water was deoxygenated by using continuous N2 bubbling for an adequate period of time (more than 6 h). The pretreated steel samples were hung separately in a high temperature and high pressure autoclave. The distilled water was pumped into the autoclave till all samples were submerged in the solution. N2 was bubbled from the bottom of the autoclave for 1 h to further ensure that it was oxygen-free. CO2 was introduced under pressure into the autoclave to maintain a total pressure of 8 MPa, and the temperature was increased to 90 °C. The corrosion film was prepared after 72 h.Exposure and electrochemical testing
The samples were taken out of the autoclave and respectively exposed to three types of solution: (1) 0.5 mol L−1 NaCl, (2) 0.5 mol L−1 Na2SO4 and (3) 0.5 mol L−1 NaHCO3. All the solutions were prepared using analytical grade reagents and distilled water and were deoxygenated sufficiently with N2 bubbling before the immersion experiment. The experiment was carried out in an airtight five necked flask at 90 °C. After 72 h exposure, the Mott–Schottky electrochemical technique and potentiodynamic polarization were carried out under the three conditions. A classic three electrode cell, including a platinum plate counter-electrode, a saturated calomel reference electrode inserted in a electrolytic bridge and a specimen of film-covered N80 steel (only 10 × 10 mm2 surface exposure), was built for the electrochemical tests. An Autolab Model PGSTAT302N electrochemical potentiostat was used to carry out electrochemical measurements. The Mott–Schottky curve was measured at a frequency of 1 kHz and with a potential scan rate of 10 mV s−1. The potentiodynamic polarization was carried out using a scan rate of 0.5 mV s−1. EIS measurements were carried out in the 100 kHz to 0.00001 kHz frequency range at open potential, with a peak-to-peak amplitude of 10 mV.SEM and XRD measurements
The phase composition analysis of the prepared film was carried out using a DX-2000 Rigakudmax X-ray diffractometer with a copper Kα X-ray source. The scanning range of 2θ started from 10° to 80°. A JSM-6490LV scanning electron microscope was used to observe the microstructure of the film.3. Results and discussion
Prepared FeCO3 corrosion film
A pure FeCO3 corrosion film was prepared successfully in distilled water with dissolved CO2 as expected, which was proven by XRD as shown in Fig. 1. All the peaks indicated that FeCO3 (siderite) was the sole product. The formation of FeCO3 is regarded as the secondary product of the anodic and cathodic electrochemical reactions when the concentrations of Fe2+ and CO32− exceed the solubility limit in the local space near the surface of steel. In the various formation solutions, the ions hardly take part in the sedimentary film directly, except for Ca+.1,18 Both CaCO3 and FeCO3 belong to the calcite structure, so the trace Ca displaces an equivalent number of Fe in the crystal lattice of FeCO3 to produce (Fe, Ca)CO3. This perturbation cannot change the properties of the corrosion film. Therefore, the pure FeCO3 film certainly represents the corrosion process in the formation solution environment. | ||
| Fig. 1 XRD diffraction of the corrosion film prepared from water with dissolved CO2 at 90 °C and 8 MPa. | ||
As can be seen in Fig. 2, the corrosion film was composed of rhombic crystal grains. A compact structure was observed, because the crystal grains are arranged in an ordered fashion. The porosity of the film is very low (below 0.1%).18
Physicochemical mechanism for semiconductor type
Mott–Schottky curves of FeCO3 films in the three solutions are shown in Fig. 3, according to the dependence of E and C−2. The electronic conducting behavior can be analyzed from the Mott–Schottky plots.A marked difference was observed from the three plots in Fig. 3. In NaHCO3 solution, a positive slope was found, which indicated an n-type semiconductor film. However, a p-type semiconductor film was judged from the negative slope in both NaCl and Na2SO4 solutions. Thus, the semiconducting properties of the FeCO3 film are determined by the anion species in the formation solution.
According to the electron band theory of a solid, if the number of electrons in the conduction band is more than that of holes in the valence band, the solid should be considered as an n-type semiconductor. In the opposite case, it belongs to the p-type semiconductors. The n-type semiconductor FeCO3 film is attributed to the doping of an interstitial cation or anion vacancy. The doping of cation vacancies in the FeCO3 film makes it present p-type semiconductor characteristics.
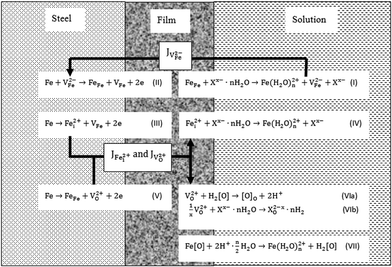
Referring to the PDM resulting from the research group of Macdonald,19 the physicochemical process of the FeCO3 films in different solutions is described schematically in Fig. 4. PDM-II is suitable for explaining the corrosion film by precipitation of ferrous ions with carbonate ions. The semiconducting properties of a FeCO3 film are determined by the complex process of generation, diffusion and annihilation of charge carriers.
It is well known that seven basic reactions occur in the steel/film/solution system, as seen in Fig. 4 labeled as (I) to (VII). The various anions have different influences on these reactions by their diverse adsorptive and reactive abilities. Reactions (I), (IV), (VI) and (VII) are present at the interface of the outer layer of the FeCO3 film and solution (film/solution), while the other reactions occur at the interface of the steel and the inner layer of the FeCO3 film (steel/film). Here, the symbols are cation vacancy, VFe2−, anion vacancy, VO2+, interstitial cation, Fei2+, neutral vacancy, VFe, ferrous ion in a cation site, FeFe, anion in anion site, [O]o, hydration anion, Xx−·nH2O, film forming anion, [O], aqueous ferrous ion, Fe(H2O)n2+, and film compound, Fe[O].
The VFe2− is generated according to reaction (I) by adsorption of Xx−·nH2O on the active FeFe in the FeCO3 film. Cation vacancies are generated by the catalysis of adsorbed species.20 The subsequent step is the diffusion of VFe2− from film/solution towards to steel/film. The VFe2− is eliminated in reaction (II). The VO2+ and Fei2+ are produced by reactions (III) and (V). After diffusion to the surface of the film, they disappear by reactions (IV) and (VI). Reaction (VII) means the dissolution of the film.
Guo et al. believed that FeCO3 had strong adsorption to anions via Coulombic as well as Lewis acid–base interactions.21 Among HCO3−, SO42− and Cl−, the former is softer than the latter two in Lewis acidity on the basis of the “hard and soft acid and base” concept,22 so the adsorption ability of HCO3− for the same base, Fe2+, in the film is weaker than SO42− and Cl−. Literature has reported that Cl− and SO42− played an important role in the corrosion products of iron to form complex compounds.23,24 It could be supposed that transient products FeCO3Cl−ads and FeCO3SO42−ads are present in the film due to the adsorption of Cl− and SO42− on FeFe in the film, where the subscript “ads” means adsorption state. Using XPS, the adsorption on the film was found to occur by M–O–X bonds.25 The amount of VFe2− caused by reaction (I) in NaHCO3 solution is much less than in Na2SO4 and NaCl solutions.
The kinetics of reaction (IV) are also affected by the Xx−·nH2O; thus, the amount of consumed Fei2+ in the Na2SO4 and NaCl solutions is more than in the NaHCO3 solution. The annihilation of VO2+ runs in two ways as reaction (VIa) and reaction (VIb). Reaction (VIa) indicates that the anion, HCO3−, is in favor of FeCO3 film formation, while reaction (VIb) cannot precipitate solid production by anions, such as SO42− and Cl−. The adsorbed anion Xx− will be trapped by VO2+. The anion occupies the VO2+, leading to further reactions. When it meets with VFe2−:
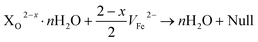 | (4) |
 | (5) |
Therefore, HCO3− takes part in the film formation, resulting in a more dense structure. On the other hand, adding NaHCO3 elevates the pH of the solution because of its strong base and weak acid performance, which mitigates the solubility of iron carbonate. However, SO42− and Cl− lead to a porous structure based on the soluble ion Fe(H2O)n2+ produced in several reactions. The anions adsorbed on the film undergo hydrolysis and reduce the local pH, which also causes the dissolution of the film. The microstructure can be confirmed from Fig. 5. The SEM images of the FeCO3 film surface after 72 h immersion in NaHCO3, Na2SO4 and NaCl solutions are shown in Fig. 5. The shape and arrangement of the crystals in the NaHCO3 solution were similar to the initial FeCO3 film prepared from distilled water with dissolved CO2. However, the surface morphology became porous after immersion in the other two solutions, and the crystalline grain changed to spherical.
Cl− is a well known corrosion accelerator of metal in aggressive environments. The role of SO42− is not well clarified. It has been found that SO42− is more aggressive than Cl−, and SO42− was the species responsible for a pitting attack on copper.26 During the formation of artificial steel rust particles prepared from acidic aqueous Fe(III) solution, the influence of SO42− is more important than Cl−.24 Deng et al. suggested that SO42− would supplant the adsorbed Cl− and accelerate the effect of pitting initiation.27 Thus, SO42− is indeed a strongly aggressive anion to metal.
In NaHCO3 solution, less VFe2− is produced, so it is easy to meet the requirement of flux:
 | (6) |
The FeCO3 film is doped by Fei2+ and VO2+, and thus it presents n-type semiconductor characteristics. In Na2SO4 and NaCl solutions, it is easy to generate VFe2−. Contrarily, the flux is abided by:
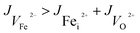 | (7) |
The FeCO3 film is a p-type semiconductor doped by VFe2−.
The charge carrier concentration Nq, flat band potential EFB and semiconductor type corresponding to each of the three solutions are listed in Table 1, where Nq includes donor density (ND) for the n-type semiconductor and acceptor density (NA) for the p-type semiconductor. All the parameters were calculated using linear fitting of the Mott–Schottky curves in Fig. 3, based on eqn (2) and (3).
| Solution | N q (cm−3) | E FB (VSCE) | Semiconductor type |
|---|---|---|---|
| NaHCO3 | 0.27 × 1023 | −0.31 | n |
| Na2SO4 | 1.72 × 1023 | −0.26 | p |
| NaCl | 17.2 × 1023 | 0.14 | p |
When the n-type or p-type semiconductor contacts the electrolyte, a conductivity band bending occurs in the semiconductor due to non-uniform distribution of charge at the interface zone. It can be described by EFB. The EFB is clearly environment-dependent. The EFB of the n-type semiconductor in NaHCO3 solution is more negative than that of the p-type semiconductor in Na2SO4 solution and NaCl solution.
The charge carrier concentration reflects the number of defects in the film. It further affects the conductivity of the film due to the transport of charge carriers. The lowest ND, 0.27 × 1023 cm−3, was obtained in the NaHCO3 solution. NA values of 1.72 × 1023 cm−3 and 17.2 × 1023 cm−3 were found in the Na2SO4 solution and NaCl solution, respectively. The lower NA in Na2SO4 solution should be due to the greater radius of SO42− than Cl−.
This indicated a less-doped semiconductor structure of the film in NaHCO3 solution and a higher doping degree of the film in the other two solutions. The electron passes through the film by the way of flow of doping ions. The higher the doping degree of the film, the better the conductivity obtained for the film.
Fig. 6 shows the XRD results of the film after immersion in the three solutions. As can be seen, the composition is still FeCO3 (siderite) in each case. According to the mechanism mentioned above, SO42− and Cl− play the role of producing charge carriers and dissolving the solid film. They do not participate directly in the substance exchange reaction. For this reason, no matter what the formation solution type in the wells containing CO2, the corrosion scale is mainly composed of FeCO3. The diffraction peak height of the film in the NaHCO3 solution is stronger than the other two. This means that the crystal perfection in the NaHCO3 solution is the best, which is clearly observed in Fig. 5.
Relation of semiconducting properties and corrosion resistance
Oliveira et al. reported that the film gave rise to higher protection when the film changed from a p-type to n-type conductor.13 Different pitting susceptibilities were obtained for the p-type and n-type semiconducting oxide films on the surface of stainless steel.28 In fact, there should not be a direct relationship between semiconductor type and corrosion resistance. The corrosion behaviors rely on two important factors, namely, potential and current. They correspond to flat band potential and charge carrier concentration.Potentiodynamic polarization was used to determine the corrosion resistance behaviors of the FeCO3 films after immersion in different solutions. The polarization curves are shown in Fig. 7, and the analysis results are shown in Table 2. After strong polarization, a high stable passivity was observed in the NaHCO3 solution due to the wide passivation potential range. However, activation polarization was presented when a high potential was applied in the Na2SO4 and NaCl solutions, that is to say, passivity was broken down by the aggressive anions Cl− and SO42−. Using the method of Tafel extrapolation, the corrosion currents were calculated from the plots in Fig. 7. They increase with in sequence of NaHCO3, Na2SO4 and NaCl solutions. Therefore, HCO3− inhibits the CO2 corrosion of N80 steel, but Cl− and SO42− lead to low corrosion resistance. Thus, the protection of the FeCO3 film in different solutions decreases in the sequence of NaHCO3, Na2SO4 and NaCl. During the electrochemical corrosion process, the anodic reaction, steel dissolution, occurs at the steel/film interface, but the cathodic reaction is always found at the film/solution interface, because the film has a more positive potential than the steel itself.29 The link between the cathodic reaction and anodic reaction is an electron, i.e., the charge carrier in the film. A higher charge carrier concentration implies a more rapid electron transfer step. The lowest charge carrier concentration results in passivation in the NaHCO3 solution. The charged particles do not diffuse easily. The higher charge carrier concentration decreases the IR (the potential drop of scale caused by flow current (I) and resistance (R) of the film) of the film, which accelerates the kinetics of corrosion in Na2SO4 and NaCl solutions.
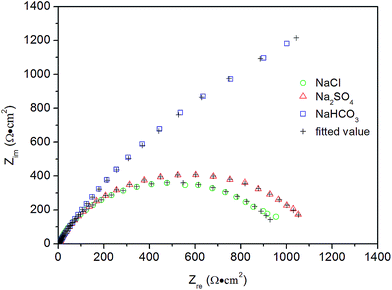
Fig. 8 shows the Nyquist plots of the EIS and their equivalent circuits in the three solutions. In the Na2SO4 and NaCl solutions, one capacitive loop (semi-circle) was observed, but a Warburg impedance (oblique line) overlapped with one capacitive loop in the NaHCO3 solution. Warburg impedance is attributed to the transfer resistance of the passive film to the conductive ions suggested in the PDM. One capacitive loop without Warburg impedance means that the electrochemical reaction at the interface between steel and film is the rate controlling step. The measured data were fitted well by the equivalent circuits shown. Table 3 lists the calculated values of the various electrochemical parameters. Rt, interface reaction resistance, is inversely proportional to the electrochemical corrosion rate. Therefore, the results also prove the higher protection of the corrosion film in NaHCO3 solution compared with the other two solutions.
| Solution | R s a (Ω cm2) | C dl b (Ω−1 cm−2 s−n) | n c | R t d (Ω cm2) | Z w e (Ω−1 cm−2 s−0.5) |
|---|---|---|---|---|---|
| a R s, solution resistance; b C dl, double layer capacitance; c n, frequency independent parameter value; d R t, interface reaction resistance; e Z w, Warburg impedance. | |||||
| NaHCO3 | 2.62 | 3.66 × 10−3 | 0.73 | 4047 | 3.91 × 10−3 |
| Na2SO4 | 3.79 | 1.42 × 10−3 | 0.79 | 1139 | — |
| NaCl | 2.09 | 1.55 × 10−3 | 0.80 | 999.5 | — |
Many works have attempted to discover the relationship between EFB and pitting. The apparent phenomenon that lowers EFB and mitigates the susceptibility of pitting nucleation was reported.30 The decrease in EFB increases the band gap energy and pitting potential. Pagitsas et al.31 mentioned that accumulation of VFe2− due to the incomplete annihilation in reaction (II) prevents condensation, and then a large void occurs due to the large number of VFe2−. The voids weaken the bonding strength of the film to the steel; as a result, pitting initiates. The vacancy condensation is proportional to the flux of cation vacancies.20 The adsorbates of some aggressive anions, resulting in cation vacancy generation at the deep donor level, are mainly responsible for pitting.32,33 Cl− and SO42− adsorption answer for the possible pitting.
The quantitative laws between electronic structure and general and pitting corrosion should be further put forward in the future.
4. Conclusions
In order to discover the influence of anions on the physicochemical performance of a FeCO3 corrosion film, the film, composed of rhombohedral FeCO3 crystals, was first prepared under high temperature and high pressure.After exposure to the NaHCO3 solution, the FeCO3 film presented an n-type semiconductive character according to the Mott–Schottky measurement. The flat band potential and donor density were −0.31 VSCE and 0.27 × 1023 cm−3. The compact microstructure was maintained. Warburg impedance was observed when EIS was measured. The lowest corrosion current was obtained by fitting the potentiodynamic polarization curve.
In the Na2SO4 solution, a p-type semiconductor with a flat band potential of −0.26 VSCE and acceptor density of 1.72 × 1023 cm−3 was obtained for the FeCO3 film. A loose microstructure was observed. The high conductive ion concentration elevated the corrosion current.
Similarly to the FeCO3 film in Na2SO4 solution, p-type semiconducting properties were found in the NaCl solution, but the flat band potential and acceptor density were enhanced to 0.14 VSCE and 17.2 × 1023 cm−3. Also, the integrity of the microstructure was damaged. The highest corrosion current was presented.
Acknowledgements
The authors thank financial support by the open fund (PLN1306) of the State Key Laboratory of Oil and Gas Reservoir Geology and Exploitation (Southwest Petroleum University), National Natural Science Foundation of China (51374180) and Key Lab of Material of Oil and Gas Field (X151514KCL24).References
- S. Nešić, Corros. Sci., 2007, 49, 4308 CrossRef PubMed.
- I. S. Molchan, G. E. Thompson, R. Lindsay, P. Skeldon, V. Likodimos, G. Em. Romanos, P. Falaras, G. Adamova, B. Iliev and T. J. S. Schubert, RSC Adv., 2014, 4, 5300 RSC.
- J. K. Heuer and J. F. Stubbins, Corros. Sci., 1999, 41, 1231 CrossRef CAS.
- J. B. Sun, G. A. Zhang, W. Liu and M. X. Lu, Corros. Sci., 2012, 57, 131 CrossRef CAS PubMed.
- Z. Yin, W. Zhao, Z. Bai and Y. Feng, Surf. Interface Anal., 2008, 40, 1231 CrossRef CAS.
- D. Liu, B. Qiu, Y. Tomoe, K. Bando and X. P. Guo, Mater. Corros., 2011, 62, 1153 CrossRef CAS.
- Y. Zhang, X. Pang, S. Qu, X. Li and K. Gao, Int. J. Greenhouse Gas Control, 2011, 5, 1643 CrossRef CAS PubMed.
- G. F. Lin, M. S. Zheng, Z. Q. Bai and Y. R. Feng, J. Iron Steel Res. Int., 2006, 13, 47 CrossRef CAS.
- K. Gao, F. Yu, X. Pang, G. Zhang, L. Qiao, W. Chu and X. Lu, Corros. Sci., 2008, 50, 2796 CrossRef CAS PubMed.
- J. Han and J. W. Carey, J. Appl. Electrochem., 2011, 41, 1367 CrossRef CAS PubMed.
- Z. Q. Bai, C. F. Chen, M. X. Lu and J. B. Li, Appl. Surf. Sci., 2006, 252, 7578 CrossRef CAS PubMed.
- G. A. Zhang and Y. F. Cheng, Electrochim. Acta, 2010, 55, 316 CrossRef PubMed.
- M. C. L. Oliveira, V. S. M. Pereira, O. V. Correa, N. B. Lima and R. A. Antunes, Corros. Sci., 2013, 69, 311 CrossRef PubMed.
- Z. Feng, X. Cheng, C. Dong, L. Xu and X. Li, J. Nucl. Mater., 2010, 407, 171 CrossRef CAS PubMed.
- Y. Zhang, M. Urquidi-Macdonald, G. R. Engelhardt and D. D. Macdonald, Electrochim. Acta, 2012, 69, 1 CrossRef CAS PubMed.
- J. K. Xiao, Chin. J. Geochem., 1990, 9, 169 CrossRef.
- C. Ren, X. Wang, L. Liu, H. Yang and N. Xian, Mater. Corros., 2012, 63, 168 CrossRef CAS.
- M. Gao, X. Pang and K. Gao, Corros. Sci., 2011, 53, 557 CrossRef CAS PubMed.
- D. D. Macdonald, Electrochim. Acta, 2011, 56, 1761 CrossRef CAS PubMed.
- Z. Jiang, X. Dai, T. Norby and H. Middleton, Corros. Sci., 2011, 53, 815 CrossRef CAS PubMed.
- H. Guo, Y. Li and K. Zhao, J. Hazard. Mater., 2010, 176, 174 CrossRef CAS PubMed.
- B. Ter-Ovanessian, C. Alemany-Dumont and B. Normand, Electrochim. Acta, 2014, 133, 373 CrossRef CAS PubMed.
- M. B. Nemer, Y. Xiong, A. E. Ismail and J. H. Jang, Chem. Geol., 2011, 280, 26 CrossRef CAS PubMed.
- H. Tanaka, N. Hatanaka, M. Muguruma, T. Ishikawa and T. Nakayama, Corros. Sci., 2013, 66, 136 CrossRef CAS PubMed.
- S. Ningshen, U. K. Mudali, V. K. Mittal and H. S. Khatak, Corros. Sci., 2007, 49, 481 CrossRef CAS PubMed.
- J. P. Duthil, G. Mankowski and A. Guisti, Corros. Sci., 1996, 38, 1839 CrossRef CAS.
- B. Deng, Y. Jiang, J. Liao, Y. Hao, C. Zhong and J. Lin, Appl. Surf. Sci., 2007, 253, 7369 CrossRef CAS PubMed.
- G. Bianchi, A. Cerquetti, F. Mazza and S. Torchio, Corros. Sci., 1972, 12, 49 CrossRef.
- J. Han, S. Nešić, Y. Yang and B. N. Brown, Electrochim. Acta, 2011, 56, 5396 CrossRef CAS PubMed.
- M. A. Pech-Canul, M. I. Pech-Canul, P. Bartolo-Pérez and M. Echeverrí, Electrochim. Acta, 2014, 140, 258 CrossRef CAS PubMed.
- M. Pagitsas, A. Diamantopoulou and D. Sazou, Electrochem. Commun., 2001, 3, 330 CrossRef CAS.
- S. Yang and D. D. Macdonald, Electrochim. Acta, 2007, 52, 1871 CrossRef CAS PubMed.
- Y. F. Cheng and J. L. Luo, Electrochim. Acta, 1999, 44, 2947 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |