A versatile binder-free TiO2 paste for dye-sensitized solar cells†
Abstract
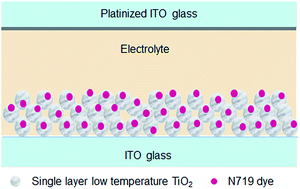
In this study, binder-free TiO2 colloidal pastes have been prepared using a variety of heterocyclic bases with diverse characteristics to produce robust photoanodes for dye-sensitized solar cells (DSSC) from a single cast film thickness of 5 micron. The influence of the base on the electrode structure and film morphology, including its electron donor characteristics are investigated after low temperature thermal treatment and high temperature sintering. The results show that quinoline in the TiO2 paste is retained within the electrode structure in comparison to piperidine and pyridine after a short thermal treatment of 150 °C for 15 minutes. The presence of organic additives with π-conjugation in the photoanode enhances both electron injection efficiency and charge carrier lifetime resulting in higher Jsc and Voc. This formulation in combination with low temperature processing yields an energy conversion efficiency of over 5% in DSSC devices. In devices where high temperature sintering is permitted, the performance of TiO2 electrodes converges towards an efficiency of over 6%, irrespective of the organic additive within the paste. This formulation offers a high degree of versatility in casting electrodes onto polymer, glass or metal foil substrates from a single source of TiO2 paste, for the many variants of low-cost solar cells.

 Please wait while we load your content...
Please wait while we load your content...