A novel sensitive fluorescent turn-on probe for rapid detection of Al3+ and bioimaging†
Abstract
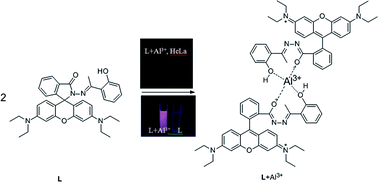
Taking advantage of the condensation reaction of 1-(2-hydroxyphenyl)ethanone and a rhodamine-B derived hydrazide, the first simply-structured rhodamine based probe L for the detection of Al3+ with high efficiency was designed and synthesized which could be applied in water solutions. Spectroscopy showed that the probe responded quickly and selectively to Al3+ over other metal ions with remarkable fluorescence enhancement (43-fold) after 10 equiv. of Al3+ were added. Sensing of Al3+ was proved to be effective over a wide pH range from 4.9 to 8.5 and the limit of the detection was calculated as 1.63 × 10−7 M by the fluorescence titration experiment. Besides, the probe L is characterized with good stability and performed perfectly in a reversibility test with EDTA. Furthermore, live-cell imaging of HeLa cells revealed the cell permeability of the probe, showing no toxicity in cultured cells by MTT method, which indicated that L could be applied in living organisms.

 Please wait while we load your content...
Please wait while we load your content...