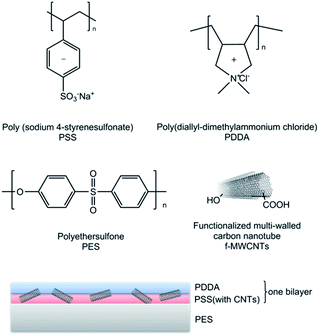
Improved antifouling performance of polyethersulfone (PES) membrane via surface modification by CNTs bound polyelectrolyte multilayers
Lei Liua,
Doris Y. W. Dia,
Hosik Parkb,
Moon Sona,
Hor-Gil Hura and
Heechul Choi*a
aSchool of Environmental Science and Engineering, Gwangju Institute of Science and Technology (GIST), 261 Cheomdan-gwagiro, 1 Oryong-dong, Buk-gu, Gwangju 500712, Republic of Korea. E-mail: hcchoi@gist.ac.kr; Fax: +82 62 7152434; Tel: +82 62 7152576
bResearch Center for Environmental Resources and Processes, Korea Research Institute of Chemical Technology (KRICT), Daejeon 305600, Republic of Korea
First published on 10th December 2014
Abstract
In this study, commercial polyethersulfone (PES) membranes were surface-modified by the deposition of functionalized carbon nanotubes (f-CNTs) bound polyelectrolyte multilayers (PEMs) through spray-assisted layer-by-layer (LbL) technique. To investigate the anti-organic fouling properties of fabricated membranes, two representative organic foulants, bovine serum albumin (BSA) and sodium alginate (SA), were selected. Single and binary organic feed solutions in the presence or absence of calcium ions were tested in cross-flow ultrafiltration apparatus. In addition, to examine the membrane resistance to bacteria fouling, the prepared membranes were immersed into Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus aureus (S. aureus) suspension for 4 hours and the adhesion of bacteria cells were observed by scanning electron microscope (SEM). The fouling and antifouling mechanisms were proposed according to the specific scenarios in this study. It was found that the enhancement of hydrophilicity and surface charge of the PES membrane mitigated organic/bio-fouling under all circumstances; the fouling and antifouling of membranes were governed by a complex interplay of interactions between foulants and membrane. Among various interactions, hydration forces and electrostatic repulsion presumably contributed significantly for reducing the adhesion of foulants. The flux of fouled membranes could be restored by the simple flushing of DI water without any chemical treatment.
1. Introduction
Membranes have played an indispensible role in water treatment for decades because of their high selectivity, small footprint, easy scale-up and environmental friendliness.1 Polymeric membranes, in particular, hydrophobic polyethersulfone (PES) membranes, are widely utilized in market due to their superior characteristics of chemical and thermal stability.2 However, commercial PES membranes are susceptible to fouling owing to their interactions with inorganic, organic or bacteria solutes through secondary forces.3,4 Fouling, caused either by inorganic compounds, organic macromolecules or bacteria, may shorten the membrane lifetime, reduce the possibility of reuse and cause extra replacement cost.5Depending on the degree of severity, fouling can be classified into reversible and irreversible fouling. Reversible fouling could be eliminated by hydraulic cleaning, such as backwashing and cross-flushing, while irreversible fouling could only be overcome by harsh chemical cleansing or by the replacement of membrane elements.6,7 Unfortunately, the fouling is irreversible and inevitable in most of the treatment applications.8 Therefore, the prevention of undesired adhesion of foulants on the membrane is an effective strategy to inhibit or mitigate adhesive fouling.9 This could be fulfilled by membrane surface modification, such as plasma treatment, polymerization or layer-by-layer (LbL) assembly. Considering the high-cost plasma equipment and stringent requirements on reaction conditions for polymerizations, the facile and tunable LbL method appears to be an attractive technique to modify membrane surface without constraints. Moreover, LbL components are not limited to polymer itself. Stabilized nanoparticles and proteins can be assembled to modify the membrane surface for enhanced hydrophilicity and charge density, so as to reduce the adhesion of foulants and impede fouling.
Although a number of studies explored the removal efficiencies of polyelectrolyte multilayer (PEM) membranes for various aqueous species, such as multi-valent ions, dyes, and organic solvents,10–12 limited researches focused on the anti-organic/bio-fouling performance of these membranes, especially nanofiller-incorporated ones. Ba et al. demonstrated the potentials of polyethylenimine (PEI)/polyacrylic acid (PAA) and PEI/polyvinyl sulfate (PVS) PEM membranes on flux restoration after being fouled by bovine serum albumin (BSA), humic acid (HA) and sodium alginate (SA).13 Tang et al. discovered that the number of E. coli deposited on poly(allylamine hydrochloride) (PAH)/PAA PEM membranes decreased by three orders of magnitude compared to polysulfone microfiltrator.14 Liu et al. reported that Ag nanoparticle-doped PAH/poly(sodium 4-styrenesulfonate) (PSS) PEM membranes posed toxic effects toward both Gram-positive B. subtilis and Gram-negative E. coli.15 Diagne et al. designed nanoAg-impregnated PSS/poly(diallyldimethyl-ammonium chloride) (PDDA) PEM membranes, and they observed E. coli inhibition and less humic acid adhesion.16
Humic substances, proteins and polysaccharides have been identified as the “culprits” for membrane organic fouling.17–19 In our previous work, PEM membranes consisting of strong acid-treated CNTs (4 wt% of polyelectrolyte) showed improved humic acid fouling resistance.20 To further examine the anti-fouling properties of this type of membrane, BSA and SA were selected as model organic foulants. In this study, the anti-bacterial characteristics of the membrane were also investigated by Gram-negative E. coli and Gram-positive S. aureus.
2. Experimental
2.1 Materials
The polyethersulfone substrate (PES-SM, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) was provided by Synder Filtration Inc., USA. Multiwalled carbon nanotubes (MWCNTs) were produced by Hanwha Nanotech., Korea (purity > 95%, diameter 10–15 nm, length 10–20 μm). Two types of polyelectrolyte, poly(sodium-4-styrenesulfonate) (PSS, Mw = 70
000 Da) was provided by Synder Filtration Inc., USA. Multiwalled carbon nanotubes (MWCNTs) were produced by Hanwha Nanotech., Korea (purity > 95%, diameter 10–15 nm, length 10–20 μm). Two types of polyelectrolyte, poly(sodium-4-styrenesulfonate) (PSS, Mw = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, powder) and poly(diallyl-dimethylammonium chloride) (PDDA, Mw = 100
000 Da, powder) and poly(diallyl-dimethylammonium chloride) (PDDA, Mw = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–200
000–200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, 20 wt% in H2O), were purchased from Sigma-Aldrich, USA, and used as received. Bovine serum albumin (BSA, powder, Sigma-Aldrich, USA) and sodium alginate (SA, powder, Sigma-Aldrich, USA) were chosen as model organic foulants without further purification. Phosphorous salts, ethanol and glutaraldehyde (GA) were obtained from Sigma-Aldrich, USA. Phosphorous buffer saline (PBS) was prepared according to the standard protocols (pH = 7.0 for anti-organic fouling test and pH = 7.4 for anti-biofouling tests). E. coli K12 (KCTC 1116) and S. aureus (KCTC 1928) were selected as model Gram-negative and Gram-positive bacteria for the anti-biofouling test. De-ionized (DI) water (Milli-Q, 18.2 MΩ cm) was used for the preparation of solutions and membrane storage.
000 Da, 20 wt% in H2O), were purchased from Sigma-Aldrich, USA, and used as received. Bovine serum albumin (BSA, powder, Sigma-Aldrich, USA) and sodium alginate (SA, powder, Sigma-Aldrich, USA) were chosen as model organic foulants without further purification. Phosphorous salts, ethanol and glutaraldehyde (GA) were obtained from Sigma-Aldrich, USA. Phosphorous buffer saline (PBS) was prepared according to the standard protocols (pH = 7.0 for anti-organic fouling test and pH = 7.4 for anti-biofouling tests). E. coli K12 (KCTC 1116) and S. aureus (KCTC 1928) were selected as model Gram-negative and Gram-positive bacteria for the anti-biofouling test. De-ionized (DI) water (Milli-Q, 18.2 MΩ cm) was used for the preparation of solutions and membrane storage.
2.2 Membrane fabrication
Prior to membrane fabrication, commercial CNTs were purified and chemically treated by sulfuric and nitric acid.2,20 The membrane fabrication process was completed with the assistance of an air pistol (SEIKI GP-1, 0.35 mm nozzle diameter, Japan), and 1 g L−1 spray solutions were prepared by individually dissolving polyelectrolytes into DI water and stirring for 4 hours. Anionic solution comprised of PSS and f-CNTs (4 wt% of PSS), whereas cationic solution only contained PDDA. The surface of vertically positioned PES substrate was alternatively deposited by anionic and cationic solution by rinsing with DI water after each deposition step. The spray and rinsing step lasted for 15 and 30 seconds, during which one minute was required for assembly process between each layer. Hereafter, the commercial PES substrate was labeled as “Mb”, the fabricated membranes were denoted as M3.5 and M6.5, where 3.5 and 6.5 represent numbers of bilayers of PSS (with f-CNTs)/PDDA deposited on PES membrane, and PSS (with f-CNTs) served as the initiating and terminating layer. The compositions of the membrane are shown in Fig. 1.2.3 Characterization of membranes
The topographical features of membranes were determined by atomic force microscopy (AFM, PSIA XE-100, Korea) with cantilever (NSC 36, Mikromasch, USA) in contact mode. The scanning area was set to 5 × 5 μm2, and at least 7 points were measured to acquire the average roughness Ra of the membrane surface. The zeta-potential measurement was performed on a zeta potentiometer (ELS-Z, Otsuka Electronics, Japan); 1 mM NaCl was used as the background electrolyte, and pH was adjusted to 7.0 by 0.1 M NaOH and 0.1 M HCl. The membranes were stored in background solution for 24 hours before measurement. Contact angles were measured by captive bubble method on a contact angle goniometer (DSA100, Krüss, Germany). The measurements were conducted at ambient temperature in DI water; 10 μL of air bubble was released from the syringe and attached on the membrane surface, and images were captured and analyzed by DSA100 software.2.4 Anti-organic fouling test
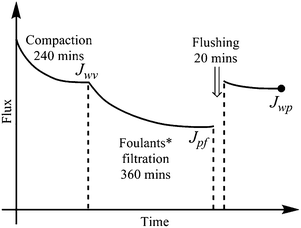
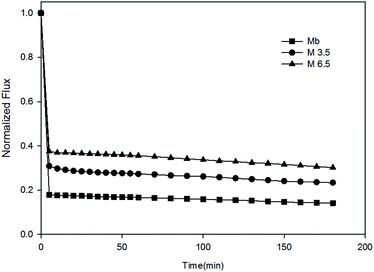
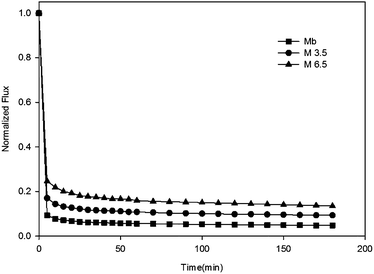
The membrane anti-organic fouling performances were evaluated by a custom-made cross-flow ultrafiltration setup, and the temperature was maintained at 25 ± 1 °C throughout the experiment by automatic temperature controller. Both permeate and retentate were circulated back to the feed tank to maintain the feed concentration. All the membranes were compacted by DI water at trans-membrane pressure (TMP) of 60 psi for 240 min, then feed solution was replaced by single or binary organic foulants in 10 mM PBS (pH = 7.0). The anti-fouling tests were performed for 360 min, followed by flux recovery test of 20 min by flushing with DI water. The scheme is illustrated in Fig. 2.The water flux J can be determined using the following formula:
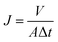 | (1) |
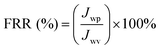 | (2) |
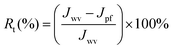 | (3) |
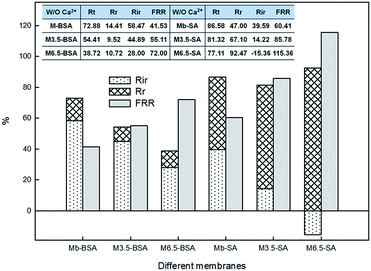
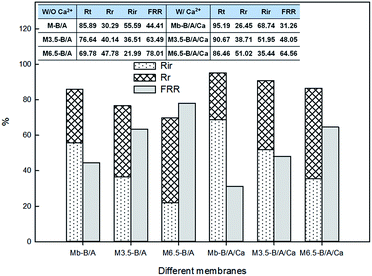
The reversible (Rr) and irreversible (Rir) ratio are defined to further differentiate factors constituting the total flux loss Rt,
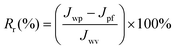 | (4) |
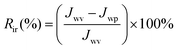 | (5) |
2.5 Bacterial anti-adhesion tests
To assess the anti-adhesion properties of membrane toward bacteria, E. coli K12 and S. aureus were first streaked on Luria–Bertani (LB) agar and brain heart infusion (BHI) agar separately and incubated overnight at 37 °C. Single colonies of bacteria were inoculated in 5 mL LB and BHI broth and incubated at 37 °C for 12 h. 100 μL of mixture was then transferred to another 5 mL liquid medium and cultured at 37 °C for 6 h to obtain viable log-phase bacteria. The bacteria in the log phase were harvested from the broth by centrifugation (Centrifuge 5418, Eppendorf) and washed by PBS (pH = 7.4) after the supernatant was decanted. The rinsing step by PBS was repeated three times to completely remove all the remaining nutrients. Optical density at 600 nm (OD600) was measured for both bacteria by one time dilution. Bacteria suspension of about 106 cells per mL of E. coli K12 and S. aureus were prepared in a final volume of 10 mL with PBS, and 1.5 × 1.5 cm2 membrane coupons of Mb, M3.5 and M6.5 were sterilized under UV irradiation for 30 min, then immersed into 10 mL of E. coli K12 and S. aureus suspension in 15 mL conical tubes and shaken at 37 °C for 4 h in an incubation shaker at 200 rpm. The membranes were withdrawn and gently rinsed with PBS. Before the SEM analysis of the adhered bacteria on the membrane surface, several steps of pretreatment were carried out as follows: the membrane coupons were immersed in 10 mL 3% (v/v) glutaraldehyde (GA) solution at 4 °C for 5 h of bacteria fixation, after washing off excess GA solution by DI water, it was dehydrated stepwise with 25%, 50%, 75% and 100% ethanol, and dried at room temperature. The bacteria on the membrane surfaces were detected by scanning electron microscopy (SEM, Hitachi S-4800, Japan).3. Results and discussions
3.1 Membrane surface properties
The contact angle measurements were performed under membrane wet state, which closely resembled real filtration conditions; therefore, the captive bubble method was considered more suitable to examine the hydrophilicity of the membrane in water treatment.21 As shown in Table 1, all the membranes were hydrophilic, the contact angle of unmodified Mb was 57°, and the quaternary ammonium together with sulfonated moieties in PEMs led to the decrease in contact angles of M3.5 and M6.5. Jones et al. and Warszynski et al. reported that the membranes capped with PSS were more hydrophilic than the ones terminated by PDDA.22,23 Furthermore, hydroxyl and carboxyl functional groups in f-CNTs further improved the hydrophilicity of PEM membranes.| Types | Contact angle (°) | Ra (nm) | ζ-potentialb (mV) |
|---|---|---|---|
| a The numbers in square brackets indicate contact angle values of PEM membrane without f-CNTs.b The values shown here are the zeta-potential values of the membranes without f-CNTs. | |||
| Mb | 57.28 ± 2.37 [null]a | 2.36 ± 0.17 | −24.15 ± 0.59 |
| M3.5 | 42.02 ± 0.91 [53.26]a | 3.10 ± 0.14 | −36.51 ± 0.54 |
| M6.5 | 39.20 ± 0.76 [48.65]a | 3.27 ± 0.19 | −52.52 ± 1.69 |
Based on the AFM results, it can be inferred that the commercial PES membrane had a relatively smooth surface. However, even f-CNTs with ∼15 nm diameter were incorporated during membrane preparation, surface modification did not induce significant change on the roughness of the membrane, which verified that the f-CNTs were embedded in the polyelectrolyte matrices by virtue of strong electrostatic interactions with PEMs. It was worth noting that the AFM analysis of membranes was carried out in a dry state, and the Ra values may vary as a result of the hydration or swelling of PEM during filtration.
The presence of electrical semiconductive CNTs in the nanocomposite interfered with the zeta-potential measurements of the membrane. The values listed in Table 1 indirectly reflected the changes of membrane surface charge by testing PEM membranes without containing f-CNTs. It was found that the negativities of the membrane surface enhanced with the deposition of more layers. We hypothesized that f-CNTs contained membranes exhibited the same behavior for zeta-potential change. Moreover, it was also expected that the negative charge densities of M3.5 and M6.5 further increased to some extent because of the hydroxyl and carboxyl groups on f-CNTs.
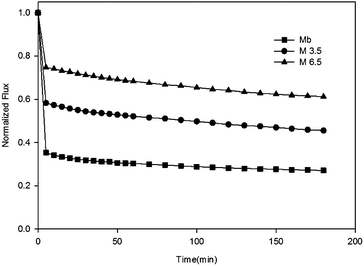
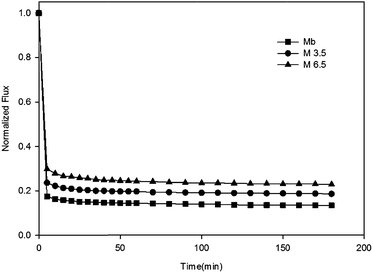
3.2 Membrane anti-fouling properties
To understand interactions between membrane surface and organic solutes, (1) filtration of single- (Section 3.2.1 and 3.2.2) and binary-solute (Section 3.2.4 and 3.2.5) feed solution and (2) subsequent surface cleaning by water flushing were investigated. Different fouling and anti-fouling profiles among membranes were distinguished by flux reduction and restoration. Moreover, the phenomena and mechanisms of anti-bacteria adhesion were studied (Section 3.2.7).Before presenting about the fouling behaviors of BSA and SA, properties of these two solutes were briefly discussed. BSA is one type of globular and flexible protein, consisting of amino acid groups in a single chain.24 At the isoelectric point (pH = 4.7), BSA bears negative charge on the surface. In contrast, SA is a linear anionic polysaccharide composed of uronic acid residues, and negative charge results from the deprotonation of carboxylic functional groups.25 Their physicochemical properties are summarized in Table 2.
| Foulants | Typical shape | MW | Carboxylic acidity |
|---|---|---|---|
| a Under pH = 7.0. | |||
| BSA | Globular | 67 kDa | 1 meq. g−1 |
| SA | Random coil | 12–80 kDa | 3.5 meq. g−1 |
| Membrane fouling scenarios | Fouling characteristics | Anti-fouling scenarios |
|---|---|---|
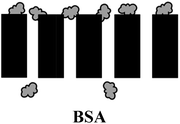
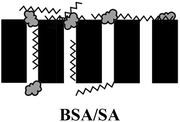
a  : BSA; : BSA;  : SA; : SA;  : calcium ion (Ca2+); : calcium ion (Ca2+);  : bacteria; : bacteria;  : PES membranes; : PES membranes;  : PEMs; : PEMs;  : f-CNTs. : f-CNTs. |
||
 |
High reversibility: electrostatic repulsion & hydration force | 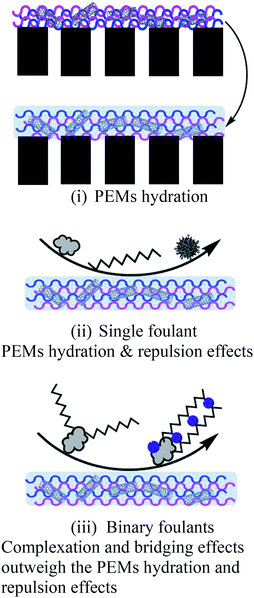 |
| Low irreversibility: pore blocking, hydrogen bonding & van der Waals forces | ||
 |
Medium reversibility: electrostatic repulsion & hydration force | |
| Medium irreversibility: hydrogen bonding & van der Waals forces | ||
 |
Medium reversibility: hydration force & electrostatic repulsion | |
| Medium irreversibility: hydrogen bonding & van der Waals forces | ||
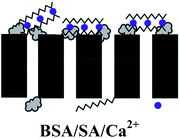 |
Low reversibility: hydration force & electrostatic repulsion | |
| High irreversibility: Ca2+ bridging, hydrogen bonding & van der Waals forces | ||
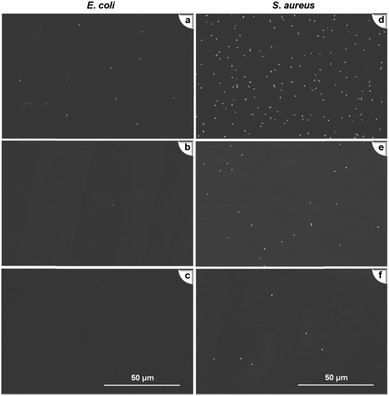 | ||
| Fig. 9 SEM images of anti-bacteria adhesion tests (a–c) are Mb, M3.5 and M6.5 for E. coli; (d–f) are Mb, M3.5 and M6.5 for S. aureus after exposure to 106 cells per mL bacteria for 4 h. | ||
The explicit contrasts were also made in bacteria adhesion on different membranes. Two types of bacteria, especially S. aureus, were entirely scattered on Mb (Fig. 9a and d), but very few dotted bacteria cells could be spotted over the PEM membranes (Fig. 9b, c, e and f). The results were consistent with the findings of Tang et al. on E. coli attachment on hydrophilic PEMs. Apart from the influence of the bacteria cell walls, Tang et al. ascribed the variations to the effects of hydrophilicity or wettability of polymeric membrane.14 For bare membrane with moderate hydrophilicity, the bacteria were withdrawn on the membrane via short-range van der Waals attraction.39 In comparison, a tightly bound water layer formed by the high hydration of the PEM membranes exerted an energetic barrier for bacteria to attach. In addition, when bacteria contacted with the membrane surface, compression of the swollen PEMs incurred steric repulsion and resisted the adhesion of bacteria simultaneously.9 The combined hydration and steric forces arose from f-CNTs bound PEM membranes, which jointly prevented bacteria attachment.
Another repulsive force worth mentioning was the electrostatic force of the PEM membrane. It inhibited bacteria-membrane interactions and added to the antifouling performance of the membranes. The f-CNTs undoubtedly improved the membrane negative charge as discussed above because most negatively charged M6.5 exhibited better anti-bacterial adhesion ability though they were slightly more hydrophilic than M3.5. Under these circumstances, the resistance to fouling was largely dependent on disparity in surface charge instead of membrane hydrophilicity. In another work, Liu et al. tested E. coli adhesion with heparin or a quaternary ammonium modified membrane and concluded that electrical interaction was more critical than membrane hydrophilicity in affecting the biofouling behaviors of membrane.40
As described in Section 3.2, membrane fouling and antifouling scenarios in this study were summarized and illustrated in Table 3. The gravity of fouling/anti-fouling could be speculated by the membrane surface properties in a single foulant system (Table 3ii); however, for mixed-foulant cases, the solute–solute and solute–ion interactions on membrane fouling need to be considered as well (Table 3iii).
4. Conclusions
In this work, the antifouling properties of surface-modified PES membrane were investigated. Through BSA and SA filtration together with flux recovery test, it was observed that the anti-organic fouling performances of PES membrane after the deposition of CNTs bound polyelectrolyte multilayers were improved. Although the presence of calcium ions unfavorably deteriorated the irreversible fouling and the gel layer may cause permanent damage to the membrane, the fabricated membranes exhibited reduced irreversible fouling compared to bare PES substrate. The resistance to bacterial adhesion was verified by E. coli and S. aureus attachment on the membrane surface, which guaranteed the prevention of biofilm formation on the resultant membranes. The prepared membranes possessed more hydrophilic and negatively charged surface, especially after the incorporation of f-CNTs, and the enhanced hydration force and electrostatic repulsion minimized the possible interactions with membrane and organic or bio-foulants, which thus reduced fouling. The current study has proved the potential application of f-CNTs bound PEM membranes in water treatment with antifouling properties.Nomenclature
| A | Effective membrane area |
| BSA | Bovine serum albumin |
| B. subtilis | Bacillus subtilis |
| E. coli | Escherichia coli |
| f-CNTs | Functionalized CNTs |
| FRR | Flux recovery ratio |
| HA | Humic acid |
| Jpf | Water flux of fouled membrane after 360 min foulant filtration |
| Jwp | Water flux of cleaned membrane after water flushing |
| Jwv | Water flux of virgin membrane |
| LbL | Layer by layer |
| CNTs | Multi-walled carbon nanotube |
| PDDA | Poly(diallyldimethyl-ammonium chloride) |
| PAA | Polyacrylic acid |
| PAH | Poly(allylamine hydrochloride) |
| PBS | Phosphorous buffer saline |
| PEI | Polyethylenimine |
| PEM | Polyelectrolyte multilayer |
| PES | Polyethersulfone |
| PSS | Poly(sodium-4-styrenesulfonate) |
| PVS | Polyvinyl sulfate |
| Rir | Flux irreversible ratio |
| Rr | Flux reversible ratio |
| Rt | Total flux loss |
| SA | Sodium alginate |
| S. aureus | Staphylococcus aureus |
| TMP | Trans-membrane pressure |
| UF | Ultrafiltration |
| V | Volume of permeated water |
| Δt | Permeation time |
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (no. NRF-2014M3C8A4030498 and upgrade R&D).Notes and references
- E. Drioli and E. Fontananova, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 395–420 CrossRef CAS PubMed.
- M. Son, H. Choi, L. Liu, H. Park and H. Choi, Environ. Eng. Res., 2014 DOI:10.4491/eer.2014.045.
- E. Celik, H. Park, H. Choi and H. Choi, Water Res., 2011, 45, 274–282 CrossRef CAS PubMed.
- R. Jamshidi Gohari, E. Halakoo, W. J. Lau, M. A. Kassim, T. Matsuura and A. F. Ismail, RSC Adv., 2014, 4, 17587–17596 RSC.
- M. F. A. Goosen, S. S. Sablani, H. Al-Hinai, S. Al-Obeidani, R. Al-Belushi and D. Jackson, Sep. Sci. Technol., 2005, 39, 2261–2297 CrossRef PubMed.
- C. Huyskens, E. Brauns, E. Van Hoof and H. De Wever, J. Membr. Sci., 2008, 323, 185–192 CrossRef CAS PubMed.
- Y. Wang, T. Wang, Y. Su, F. Peng, H. Wu and Z. Jiang, Langmuir, 2005, 21, 11856–11862 CrossRef CAS PubMed.
- H. Ma, C. N. Bowman and R. H. Davis, J. Membr. Sci., 2000, 173, 191–200 CrossRef CAS.
- V. Kochkodan, D. J. Johnson and N. Hilal, Adv. Colloid Interface Sci., 2014, 206, 116–140 CrossRef CAS PubMed.
- G. Kalaiselvi, P. Maheswari, S. Balasubramanian and D. Mohan, Desalination, 2013, 325, 65–75 CrossRef CAS PubMed.
- M. Tamaddondar, H. Pahlavanzadeh, S. Saeid Hosseini, G. Ruan and N. R. Tan, J. Membr. Sci., 2014, 472, 91–101 CrossRef CAS PubMed.
- S. U. Hong, M. D. Miller and M. L. Bruening, Ind. Eng. Chem. Res., 2006, 45, 6284–6288 CrossRef CAS.
- C. Ba, D. A. Ladner and J. Economy, J. Membr. Sci., 2010, 347, 250–259 CrossRef CAS PubMed.
- L. Tang, W. Gu, P. Yi, J. L. Bitter, J. Y. Hong, D. H. Fairbrother and K. L. Chen, J. Membr. Sci., 2013, 446, 201–211 CrossRef CAS PubMed.
- X. Liu, S. Qi, Y. Li, L. Yang, B. Cao and C. Y. Tang, Water Res., 2013, 47, 3081–3092 CrossRef CAS PubMed.
- F. Diagne, R. Malaisamy, V. Boddie, R. D. Holbrook, B. Eribo and K. L. Jones, Environ. Sci. Technol., 2012, 46, 4025–4033 CrossRef CAS PubMed.
- W. S. Ang, A. Tiraferri, K. L. Chen and M. Elimelech, J. Membr. Sci., 2011, 376, 196–206 CrossRef CAS PubMed.
- M. Beyer, B. Lohrengel and L. D. Nghiem, Desalination, 2010, 250, 977–981 CrossRef CAS PubMed.
- M. Hashino, K. Hirami, T. Katagiri, N. Kubota, Y. Ohmukai, T. Ishigami, T. Maruyama and H. Matsuyama, J. Membr. Sci., 2011, 379, 233–238 CrossRef CAS PubMed.
- L. Liu, M. Son, H. Park, E. Celik, C. Bhattacharjee and H. Choi, RSC Adv., 2014, 4, 32858–32865 RSC.
- Y. Baek, J. Kang, P. Theato and J. Yoon, Desalination, 2012, 303, 23–28 CrossRef CAS PubMed.
- R. Malaisamy, A. Talla-Nwafo and K. L. Jones, Sep. Purif. Technol., 2011, 77, 367–374 CrossRef CAS PubMed.
- M. Elzbieciak, M. Kolasinska and P. Warszynski, Colloids Surf., A, 2008, 321, 258–261 CrossRef CAS PubMed.
- P. Ying, G. Jin and Z. Tao, Colloids Surf., B, 2004, 33, 259–263 CrossRef CAS PubMed.
- K. Katsoufidou, S. G. Yiantsios and A. J. Karabelas, J. Membr. Sci., 2007, 300, 137–146 CrossRef CAS PubMed.
- B. Mi and M. Elimelech, J. Membr. Sci., 2008, 320, 292–302 CrossRef CAS PubMed.
- L. D. Nghiem, P. J. Coleman and C. Espendiller, Desalination, 2010, 250, 682–687 CrossRef CAS PubMed.
- R. Chan, V. Chen and M. P. Bucknall, Biotechnol. Bioeng., 2004, 85, 190–201 CrossRef CAS PubMed.
- S. Lee, W. S. Ang and M. Elimelech, Desalination, 2006, 187, 313–321 CrossRef CAS PubMed.
- H. Susanto, H. Arafat, E. M. L. Janssen and M. Ulbricht, Sep. Purif. Technol., 2008, 63, 558–565 CrossRef CAS PubMed.
- D. Jermann, W. Pronk, S. Meylan and M. Boller, Water Res., 2007, 41, 1713–1722 CrossRef CAS PubMed.
- F. Neemann, S. Rosenberger, B. Jefferson and E. J. McAdam, J. Membr. Sci., 2013, 446, 310–317 CrossRef CAS PubMed.
- Q. Li, Z. Xu and I. Pinnau, J. Membr. Sci., 2007, 290, 173–181 CrossRef CAS PubMed.
- M. Mänttäri, L. Puro, J. Nuortila-Jokinen and M. Nyström, J. Membr. Sci., 2000, 165, 1–17 CrossRef.
- A. Seidel and M. Elimelech, J. Membr. Sci., 2002, 203, 245–255 CrossRef CAS.
- X. Zhang, L. Wang and E. Levanen, RSC Adv., 2013, 3, 12003–12020 RSC.
- S. L. Walker, J. A. Redman and M. Elimelech, Langmuir, 2004, 20, 7736–7746 CrossRef CAS PubMed.
- M. Hermansson, Colloids Surf., B, 1999, 14, 105–119 CrossRef CAS.
- G. Speranza, G. Gottardi, C. Pederzolli, L. Lunelli, R. Canteri, L. Pasquardini, E. Carli, A. Lui, D. Maniglio, M. Brugnara and M. Anderle, Biomaterials, 2004, 25, 2029–2037 CrossRef CAS PubMed.
- C. X. Liu, D. R. Zhang, Y. He, X. S. Zhao and R. Bai, J. Membr. Sci., 2010, 346, 121–130 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |