Determination of multi-class antimicrobial residues in soil by liquid chromatography-tandem mass spectrometry
Kui Bian†
,
YaHong Liu†,
ZongNan Wang,
Tong Zhou,
XuQin Song,
FangYu Zhang and
LiMin He*
National Reference Laboratory of Veterinary Drug Residues (SCAU), College of Veterinary Medicine, South China Agricultural University, Guangzhou, Guangdong PC 510642, China. E-mail: liminokhe@scau.edu.cn; Fax: +86 20 85284896; Tel: +86 20 85280665
First published on 2nd March 2015
Abstract
Antimicrobial residues in environmental matrices may result in the occurrence of antimicrobial-resistant bacteria in soil. In this paper, a new analytical method based on liquid chromatography-tandem mass spectrometry for multiresidue analysis of 24 antimicrobials of a wide polarity range and variable physicochemical properties, including sulfonamides, tetracyclines, fluoroquinolones, macrolides, lincosamides and pleuromutilins in soil was developed. Samples were extracted with an acetonitrile: Na2EDTA–McIlvaine buffer (pH 4.0, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) system and then re-extracted with a 0.2 M sodium hydroxide solution. The extracts were purified using an HLB solid phase extraction cartridge. Chromatographic separation of the components was performed on a Zorbax SB-Aq column using acetonitrile–0.1% formic acid as mobile phase. The method developed was linear in a concentration range from the limits of quantification to 200 μg kg−1, with correlation coefficients higher than 0.99. The limits of detection and limits of quantification ranged from 0.01 to 2 μg kg−1 and 0.04 to 5 μg kg−1, respectively. The overall average recoveries for target analytes were more than 60% except for tetracycline (59.3%) in three spiked levels of 1, 4 and 20 μg kg−1 with relative standard deviations less than 20%. The method was further applied for the determination of residual antimicrobials in real samples. Some target antimicrobials were detected at different levels and tetracycline residues were dominant. 163.6 μg kg−1 of chlortetracycline was detected in a soil sample. The results indicate that the proposed method has good feasibility.
5, v/v) system and then re-extracted with a 0.2 M sodium hydroxide solution. The extracts were purified using an HLB solid phase extraction cartridge. Chromatographic separation of the components was performed on a Zorbax SB-Aq column using acetonitrile–0.1% formic acid as mobile phase. The method developed was linear in a concentration range from the limits of quantification to 200 μg kg−1, with correlation coefficients higher than 0.99. The limits of detection and limits of quantification ranged from 0.01 to 2 μg kg−1 and 0.04 to 5 μg kg−1, respectively. The overall average recoveries for target analytes were more than 60% except for tetracycline (59.3%) in three spiked levels of 1, 4 and 20 μg kg−1 with relative standard deviations less than 20%. The method was further applied for the determination of residual antimicrobials in real samples. Some target antimicrobials were detected at different levels and tetracycline residues were dominant. 163.6 μg kg−1 of chlortetracycline was detected in a soil sample. The results indicate that the proposed method has good feasibility.
1. Introduction
In recent decades, because large amounts of drugs have been used in human and veterinary medicine,1 they have been widely detected in a variety of environmental matrices such as water and soil.2 Currently, pharmaceutical residues in the environment are of increasing worldwide concern. After administration, pharmaceuticals and their metabolites are excreted by animals and humans, and then the excretion of the faeces together with urine flows into the environment. Finally, these compounds accumulate in soil. Some hydrophilic drugs may be mobile in soil which can contaminate ground water,3 and then they are introduced into the environment and even into crops and the food supply.4 So the existence of antimicrobials in water and soil may pose a risk to human health and environment ecology. In addition, the widespread use and environmental persistence of some veterinary or human drugs in the environment have raised concerns about the potential for the increase of antibiotic-resistant bacteria.5 Bacteria resistant to antimicrobials have been found in aquatic environment and soil.6,7 How to effectively assay the residues of antimicrobials in environments such as water bodies, soil and the atmosphere has become a hot topic of research.Several methods for the analysis of the commonly used antimicrobials in water,8 animal tissues,9 milk,10 and manure11 have been described using liquid chromatography-tandem mass spectrometry (LC-MS/MS). However, because of the heterogeneity of solid matrices and the great diversity of pharmaceuticals with very different polarity and functionality, the determination of antimicrobials residues in soils is poorly documented. Their presence and distribution in the soil via land application are far from being fully understood, which is primarily due to a lack of appropriate analytical methodologies. In addition, most of the available multi-extraction procedures and instrumental analytical methods for solid environmental samples cover only one12 or specific classes of antimicrobials.13,14 But none of these methods includes most common veterinary antimicrobials. Therefore, the development of a sensitive analytical method that allows for determining the residues of several classes of common veterinary drugs in soil is necessary.
The available information about the environmentally relevant concentrations of the commonly used antimicrobials is also limited; it is mostly due to analytical difficulties encountered. When trying to analyze these compounds at trace levels, various factors such as their polarity, solubility, pKa, Kow and stability in complex matrices shall be considered. As for soil matrix, the sample pre-treatment is the most difficult and time-consuming, and often involves one or more extraction and cleanup steps. Techniques of extraction such as pressurized liquid extraction (PLE),6 microwave-assisted solvent extraction (MASE)15 and supercritical fluid extraction (SFE)16 have been introduced. The common advantages of all the techniques can be referred the improvement of rapidity and automation. However, some particular drawbacks must be considered. The PLE and SFE techniques require expensive apparatus and complicated optimization procedures. The MASE technique can improve extraction efficiency, but lacks extraction selectivity, thus, and it is required for a further cleanup step. Although the MASE technique is not easily automated, it can reduce the organic solvent consumption and no specialized laboratory equipment is required. After extraction, in common, purification has to be performed by solid-phase extraction (SPE), liquid–liquid extraction (LLE), gel-permeation chromatography (GPC) or semi-preparative liquid chromatography (LC). The SPE method is often preferred since it is faster, requires less solvent and has a lower risk of sample contamination. Due to the hydrophilic–lipophilic balance (HLB) properties and the effectiveness in the extraction of a wide range of acidic, basic and neutral compounds from various matrices, Oasis HLB is one of the most widely utilized SPE sorbent for pharmaceutical extraction in soil samples. In this study, the extraction efficiencies of the C18 and MCX SPE cartridges were compared with that of the HLB SPE cartridge.
The present study focuses on developing a sensitive, selective and reproducible method for the simultaneous determination of 24 different antimicrobials including six sulfonamides (SAs), four tetracyclines (TCs), six fluoroquinolones (FQs), five macrolides (MLs), one lincosamides (LAs) and two pleuromutilins (PMs) in soils using LC-MS/MS with a triple quadrupole analyzer. Different extraction solutions, extract ratios and types of solid-phase extraction cartridges for soil sample preparation were discussed and optimized. Afterwards, the method developed was successfully applied to the determination of 100 soils samples randomly collected from different sources (35 piggeries, 25 vegetable fields, 20 living quarters, 20 orchards) in Guangdong Province, China.
2. Experimental
2.1. Reagents and materials
Reference standards of all pharmaceuticals including difluoxacin, sarafloxacin, enrofloxacin, ciprofloxacin, enoxacin, norfloxacin, chlortetracycline, oxytetracycline, doxycycline, tetracycline, sulfaquinoxaline, sulfaclozine, sulfamethoxydiazine, sulfamonomethoxine, sulfadimidine, sulfamethoxazole, tylosin, roxithromycin, kitasamycin, erythromycin, tilmicosin, clindamycin, valnemulin and tiamulin (purity > 90%) were purchased from China Institute of Veterinary Drugs Control (Beijing, China) and J & K Chemical LTD (Beijing, China). HPLC-grade methanol (MeOH), acetonitrile (ACN) and formic acid were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Ethylenedi-minetetraacetic acid disodium salt dihydrate (Na2EDTA·2H2O), sodium hydroxide pellets (NaOH), disodium hydrogen phosphate (Na2HPO4·12H2O), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O) and citric acid monohydrate (H3Cit·H2O), hydrochloric acid (HCl, 37%, w/v) and ammonia solution (25%, w/v) were purchased from the Guangzhou Chemical Reagent Company (Guangzhou, China). Ammonium acetate was purchased from TEDIA (Fairfield, OH, USA). Deionized water was obtained using a Millipore purification system Milli-Q (Molsheim, France). Other chemical reagents were of analytical reagents grade.Oasis HLB (hydrophilic–lipophilic balance, poly(divinylbenzene-co-N-pyrrolidone), 60 mg, 3 mL) SPE cartridge and Oasis MCX SPE cartridge (60 mg, 3 mL) were purchased from Waters Co. (Milford, MA, USA). Bond Elut-C18 SPE cartridge (200 mg, 3 mL) was purchased from Agilent Technologies Co. (Santa Clara, CA, USA).
A Na2EDTA–McIlvaine buffer solution (0.1 M) was prepared by mixing 1000 mL of 0.1 M citric acid with 625 mL of 0.2 M disodium hydrogen phosphate (pH adjusted to 4.0 ± 0.05 with NaOH or HCl as needed), and then 60.5 g of Na2EDTA·2H2O was added into the above mixture.
Individual stock solutions were prepared at concentrations of 100 mg L−1 in methanol and stored at −20 °C. Mixed working standard solutions were prepared by the adequate mixing and dilution of the individual stock solutions.
2.2. Sample preparation and extraction
Blank soil sample selected for the establishment of the quantitative method was collected from a livestock farm at a depth of 0–10 cm. Soil samples were passed through a 3 mm sieve to remove plant detritus, root and gravel, and then stored at −20 °C until further analysis.A sieved soil sample (5.0 g) was introduced into a 50 mL polypropylene centrifuge tube and spiked at 1, 4 and 20 μg kg−1 by the addition of 100 μL appropriate mixed working solutions. After being stand at least 20 min, 15 mL of extraction buffer (ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2EDTA–McIlvaine buffer (pH 4.0, 5
Na2EDTA–McIlvaine buffer (pH 4.0, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v)) were added into the tube. The tube was vortex mixed to achieve homogeneity, and then the tube was ultrasonicated for 10 min, shaken for 20 min, finally centrifuged at 9000 rpm for 10 min. The supernatant was transferred to clean glassware and evaporated to below 7 mL in 45 °C water bath. The soil residue was extracted with 10 mL of 0.2 M NaOH again. The top aqueous layer was decanted to a new tube, adjusted pH to 4.0 with 1 M HCl, and centrifuged at 6000 rpm for 5 min. All the supernatant were combined prior to the cleanup step by solid phase extraction.
5, v/v)) were added into the tube. The tube was vortex mixed to achieve homogeneity, and then the tube was ultrasonicated for 10 min, shaken for 20 min, finally centrifuged at 9000 rpm for 10 min. The supernatant was transferred to clean glassware and evaporated to below 7 mL in 45 °C water bath. The soil residue was extracted with 10 mL of 0.2 M NaOH again. The top aqueous layer was decanted to a new tube, adjusted pH to 4.0 with 1 M HCl, and centrifuged at 6000 rpm for 5 min. All the supernatant were combined prior to the cleanup step by solid phase extraction.
2.3. Solid phase extraction
Cleanup and enrichment were performed on the Oasis HLB cartridge, which was conditioned using 3 mL methanol followed by 3 mL ultrapure water and 3 mL Na2EDTA–McIlvaine buffer. The supernatant was loaded into the cartridge at approximate 1 mL min−1. The cartridge was then washed with 6 mL of 5% methanol in water and dried by applying a low positive pressure for 2 min, eventually the analytes were eluted with 6 mL methanol. The eluate was evaporated to near dryness under gentle nitrogen flux at 45 °C, and then re-dissolved in 1.00 mL of 20% methanol in 0.1% formic acid solution prior to analysis by LC-MS/MS.2.4. LC-MS/MS analysis
The chromatographic system was composed of an Agilent 1200 series high-performance liquid chromatography (HPLC) system, including quaternary pump and autosampler (Milford, MA, USA). The mass system included Applied Biosystems API 4000 triple quadrupole mass spectrometer with electrospray ionization (ESI) interface and Analyst 1.5 software (Foster City, CA, USA).Chromatographic separation was performed using an Agilent Zorbax SB-Aq C18 column (150 mm × 2.1 mm i.d., 3.5 μm). The mobile phase consisted of acetonitrile (A) and 0.1% formic acid in water (B). The mobile phase used in the gradient elution consisted of solvent A and solvent B. As described in our previous study,26 the linear gradient developed for the analysis was performed as follows: 0–0.2 min 10% A; 0.2–1.0 min 10–20% A; 1.0–11 min 20–40% A; 11–15 min 40–90% A; 15–16 min 90% A; 16–18 min 90–10% A; 18–26 min 10% A. The total runtime was 26 min. The column was maintained at 35 °C. The flow rate was 0.2 mL min−1 and the injection volume was 5 μL.
The tandem MS analyses were carried out on API 4000 triple quadrupole mass spectrometer with electrospray ionization source. The turbo ion-spray source was used in positive mode with the following settings: ion spray voltage (IS), 5000 V; ion source temperature, 600 °C; dwell time, 50 ms. The optimal collision energy (CE), declustering potential (DP) and transitions chosen for the multiple reaction monitoring (MRM) are listed in Table 1. Acquisition and analysis of data were performed through Analyst 1.5 software (Applied Biosystems) in Windows XP platform-based data-processing system.
| Compounds | Abbr. | Precursor ion [M + H]+ | Product ion | DP (V) | CE (eV) | Rt (min) | Compounds | Abbr. | Precursor ion | Product ion | DP (V) | CE (eV) | Rt (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Abbr., abbreviations; DP, declustering potential; CE, collision energy; Rt, retention time.b For identification. | |||||||||||||
| Fluoroquinolones | FQs | Sulfamethoxydiazine | SMD | 281.2 | 156 | 60 | 25 | 11.7 | |||||
| Difluoxacin | DIF | 400.4 | 382.3 | 60 | 28 | 12.1 | Sulfamonomethoxine | SMM | 281.2 | 215.1b | 60 | 25 | 12.7 |
| 356.2b | 28 | 156 | 25 | ||||||||||
| Sarafloxacin | SAR | 386.4 | 368.2 | 60 | 28 | 11.8 | Sulfadimidine | SM2 | 279.2 | 215.1b | 60 | 26 | 10.6 |
| 342.3b | 28 | 186 | 25 | ||||||||||
| Enrofloxacin | ENR | 360.6 | 316.4 | 60 | 30 | 10.7 | Sulfamethoxazole | SMZ | 254.2 | 156b | 53 | 28 | 13.7 |
| 245.1b | 37 | 156 | 23 | ||||||||||
| Ciprofloxacin | CIP | 332.4 | 314.2 | 60 | 25 | 9.9 | Macrolides | MLs | 91.7b | 40 | |||
| 288.3b | 25 | ||||||||||||
| Enoxacin | ENO | 321.1 | 303.2 | 63 | 28 | 9.4 | Tylosin | TYL | 916.6 | 174.3 | 101 | 52 | 16.1 |
| 234.2b | 28 | 772.6b | 41 | ||||||||||
| Norfloxacin | NOR | 320.4 | 302.3 | 50 | 26 | 9.6 | Roxithromycin | ROX | 837.8 | 679.5 | 60 | 33 | 17.6 |
| 276.6b | 16 | 158.2b | 55 | ||||||||||
| Tetracyclines | TCs | Kitasamycin | KIT | 772.4 | 109.1 | 90 | 78 | 17.7 | |||||
| Chlortetracycline | CTC | 479.3 | 444.2 | 71 | 29 | 11.5 | Erythromycin | ERY | 734.7 | 174.2b | 64 | 50 | 14.8 |
| 462.1b | 24 | 158 | 43 | ||||||||||
| Oxytetracycline | OTC | 460.7 | 426.1 | 65 | 26 | 8.7 | Tilmicosin | TIL | 869.6 | 576.5b | 130 | 27 | 12.8 |
| 443.3b | 17 | 696.4 | 66 | ||||||||||
| Doxycycline | DC | 445.2 | 410.2 | 65 | 27 | 9.5 | Lincosamides | LAs | 174.2b | 60 | |||
| 427.2b | 19 | ||||||||||||
| Tetracycline | TC | 445.2 | 428.2 | 70 | 25 | 12.2 | Clindamycin | CLI | 425.2 | 126.2 | 72 | 37 | 11.9 |
| 153.9b | 44 | 377.3b | 27 | ||||||||||
| Sulfonamides | SAs | Pleuromutilins | PMs | ||||||||||
| Sulfaquinoxaline | SQ | 301.3 | 156 | 62 | 24 | 16.5 | Valnemulin | VAL | 565.5 | 263.1 | 80 | 25 | 18.1 |
| 91.7b | 44 | 164.2b | 44 | ||||||||||
| Sulfaclozine | SCZ | 285.2 | 155.9 | 60 | 23 | 16.1 | Tiamulin | TIA | 494.5 | 192.2 | 48 | 29 | 17.3 |
| 107.7b | 38 | 119.2b | 55 | ||||||||||
2.5. Method validation
The performance characteristics of the developed method including selectivity, limit of detection (LOD), limit of quantification (LOQ), recovery and precision were evaluated.The selectivity of the method was checked by analyzing 50 blank soil samples from different sources to evaluate possible matrix interferences. The results were evaluated by the presence of interfering substances around the analyte's retention time.
Linearity was evaluated by using of matrix-matched calibration curves. Seven-point ranging from the LOQ of each analyte to 200 μg kg−1 was prepared by spiking corresponding amounts of target compounds into five gram blank soil extracts.
The LOD and LOQ for the analyte in soil were determined by signal to noise ratio (S/N) of 3 and 10, respectively. The most common method was based on the chromatographic response regarding the most intense ion transition for quantification and the ion transition ratio used for confirmation.
Recoveries and precision for the entire method were evaluated by spiking blank soil samples at three concentration levels (low, 1 μg kg−1; medium, 4 μg kg−1; and high, 20 μg kg−1) for target analytes in six replicates at each level for three consecutive days. The recoveries of twenty-four analytes at the spiked samples were calculated by measuring the ratios of the predicted value obtained from the matrix-matched calibration curves to the corresponding spiked values. Intra-day precision was determined for the three concentration levels in six replicates for each concentration on the same day. Inter-day precision was determined for the three concentration levels in six replicates for each concentration on three different days. The intra-day and inter-day precisions were estimated by calculating the relative standard deviation (RSD, %) for the different concentrations.
Stability was expressed as a percentage of the initial value. Due to the significant difference of physicochemical properties of the 24 antimicrobials, the stability in pure solvent and sample solution should be checked prior to chromatographic investigations. This research mainly investigated the stability of the stock solution of the target analytes under −20 °C within 30 days and the short-term stability of the soil sample including room temperature (25 °C, in the autosampler) and 4 °C within 6, 12, 24 and 48 h. All stability studies were conducted in triplicate. The measured values were compared with those freshly prepared pure solvent and matrix standard solutions at different concentrations.
2.6. Matrix effects (ME)
Matrix effects are common in LC-MS/MS analysis due to the molecules co-elute with the compounds of interest and alter their ionization efficiency in the ionization interface, causing ion suppression or enhancement.14 The intensity of matrix effect was evaluated by the method of post-extraction addition.17 The percentage of ME is calculated as| ME (%) = B/A × 100 |
3. Results and discussion
3.1. Sample extraction
In order to develop an effective sample extraction step, several extraction solvents including its volume and ratio of the buffer in solvent system were evaluated.Many minerals and organic matter in the soil matrix may form kinds of interactions (such as complexation, hydrogen bonding, hydrophobic interaction and ion-exchange) with the analytes, so that the extraction of the compounds of interest from soil becomes difficult and complex. Therefore, an appropriate sample pretreatment method is very important for an accurate determination of target analytes in soil samples. On basis of the physicochemical properties of the target compounds and the extraction approaches of similar sample matrix in literatures,5,13,14,18,19 several preliminary experiments were performed to extract the antimicrobials residues from soil samples. Thus the following five extraction solvent systems were tested:
- M1 = ACN/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
- M2 = ACN/acetate buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 4.0).
1, v/v, pH 4.0).
- M3 = ACN/acetate buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 4.0) and 0.5 g Na2EDTA.
1, v/v, pH 4.0) and 0.5 g Na2EDTA.
- M4 = ACN/citrate buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 4.0) and 0.5 g Na2EDTA.
1, v/v, pH 4.0) and 0.5 g Na2EDTA.
- M5 = ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2EDTA–McIlvaine buffer (5
Na2EDTA–McIlvaine buffer (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v, pH 4.0).
5, v/v, pH 4.0).
Blank soil samples were spiked with 100 μL of 0.2 mg L−1 (each) mixed working standard solution to evaluate the mean recoveries based on the mentioned extractive method above. The recoveries are summarized in Fig. 1. The results demonstrate that good yields (more than 80%) were obtained only for SAs, clindamycin, roxithromycin and tiamulin when using the M1 system, however, the recoveries of the other compounds were very low (most analytes less than 20%). Salvia et al.5 suggested that the acetate-based method could result in better recoveries, particularly for veterinary antimicrobials such as sulfonamides and macrolides. Therefore, the M2 and M3 systems were also chosen as the extraction solvent. The results show that the high recoveries (70% above) were obtained for major target analytes such as SAs, MLs and LAs. However, the measured recovery ratios of 4 TCs and 6 FQs were all below 60%, and the recoveries of the ten analytes obtained by the M2 were slightly lower than those by the M3 (the addition of Na2EDTA).
TCs and FQs have a strong adsorption capacity to the soil since the polarity/ionic functional groups existed in their chemical structures. So for improving the extraction efficiency of TCs and FQs from soil samples, a complexation agent (Na2EDTA buffer and (or) citrate buffer), which can abate the chelate effect, was added to avoid the complexation of these analytes with divalent cations such as Mg2+ or Ca2+ in soil20 and facilitate the extraction of bound compounds. As shown in Fig. 1, the recovery ratios of five of the six FQs (except difloxacin) and one (tetracycline) of the four TCs were below 40% when the M4 system was used as the extraction solvent. In contrast, the M5 system achieved relatively high recoveries for all the analytes except FQs (12–36%). Thus, the M5 could be used to extract most target analytes from soil samples. Further, the volume ratio of ACN in the Na2EDTA–McIlvaine buffer (for example, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5
3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 3
5 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) was investigated. The experiments show that the recoveries of most analytes (except for FQs) increased with the decrease of acetonitrile in the extraction solvent. The higher recoveries (more than 60%) were obtained with the 5
5, v/v) was investigated. The experiments show that the recoveries of most analytes (except for FQs) increased with the decrease of acetonitrile in the extraction solvent. The higher recoveries (more than 60%) were obtained with the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio of ACN to Na2EDTA–McIlvaine buffer than both the 9
5 ratio of ACN to Na2EDTA–McIlvaine buffer than both the 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 7
1 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. However, too low ACN (3
3. However, too low ACN (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) in M5 system resulted in low recoveries for MLs and PMs. Several volumes of the M5 system (10, 15 and 20 mL) were subsequently tested. The results indicate that the volume of 15 mL gave higher recoveries than the volume of 10 mL, especially for TCs. On the other hand, compared to 15 mL, the 20 mL did not significantly increase the recoveries for most of the analytes. Therefore, in order to get the higher recoveries, while minimizing the consumption of solvent and time, the volume of 15 mL M5 was chosen for the following experiments.
5, v/v) in M5 system resulted in low recoveries for MLs and PMs. Several volumes of the M5 system (10, 15 and 20 mL) were subsequently tested. The results indicate that the volume of 15 mL gave higher recoveries than the volume of 10 mL, especially for TCs. On the other hand, compared to 15 mL, the 20 mL did not significantly increase the recoveries for most of the analytes. Therefore, in order to get the higher recoveries, while minimizing the consumption of solvent and time, the volume of 15 mL M5 was chosen for the following experiments.
For enhancing the recoveries of FQs, further optimization of extraction protocols was needed. According to the properties of these compounds and the corresponding literatures on the analysis of FQs residues, several extraction solvents including acidic, basic and different buffer solutions were evaluated. Blank soil samples were spiked with 100 μL of 0.2 mg L−1 (each) mixed working standard solution to evaluate the extraction recoveries of different solvents. The results are summarized in Table 2. The pH value of the extraction solvent had a great influence on the extraction efficiency of FQs. 0.1 M HCl, 0.05 M orthophosporic acid and 5% formic acid in acetonitrile did not extract any FQs. The phosphate buffer (pH 3.2)–acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) system and phosphate buffer (pH 7.4) also gave very poor recoveries (all below 40% for the six FQs). Delepine et al.21 used 0.05 M phosphate buffer solution (pH 7.4) to extract FQs from muscle. Good recoveries for FQs were obtained. But in our experiments, perhaps because there are a great number of divalent metallic elements and organic matters in soil matrix, very low recoveries were obtained when the phosphate buffer solution was used to extract FQs from soil. Turiel et al.22 reported that the high recoveries for FQs could be obtained when the 50% (w/v) Mg(NO3)2 solution containing 4% of ammonia was used to desorb and extract FQs from soil on basis of the formation of fluoroquinolones–Mg2+ complexes. In this study, good recoveries were also obtained using this extraction solution. Nevertheless, because Mg2+ in the extracts formed precipitation with the Na2EDTA–McIlvaine buffer solution, resulting in blockage of the SPE cartridge in the cleanup step. Fortunately, good recoveries for FQs were achieved when using strong basic solution as an extraction solvent. One reason was due to FQs (as anionic form) being dissolved in sodium hydroxide solution. Another reason was that in alkaline condition the carboxyl of FQs was negatively charged, which has an electrostatic repulsion to the negative charge on the surface of the soil.
1, v/v) system and phosphate buffer (pH 7.4) also gave very poor recoveries (all below 40% for the six FQs). Delepine et al.21 used 0.05 M phosphate buffer solution (pH 7.4) to extract FQs from muscle. Good recoveries for FQs were obtained. But in our experiments, perhaps because there are a great number of divalent metallic elements and organic matters in soil matrix, very low recoveries were obtained when the phosphate buffer solution was used to extract FQs from soil. Turiel et al.22 reported that the high recoveries for FQs could be obtained when the 50% (w/v) Mg(NO3)2 solution containing 4% of ammonia was used to desorb and extract FQs from soil on basis of the formation of fluoroquinolones–Mg2+ complexes. In this study, good recoveries were also obtained using this extraction solution. Nevertheless, because Mg2+ in the extracts formed precipitation with the Na2EDTA–McIlvaine buffer solution, resulting in blockage of the SPE cartridge in the cleanup step. Fortunately, good recoveries for FQs were achieved when using strong basic solution as an extraction solvent. One reason was due to FQs (as anionic form) being dissolved in sodium hydroxide solution. Another reason was that in alkaline condition the carboxyl of FQs was negatively charged, which has an electrostatic repulsion to the negative charge on the surface of the soil.
| Solvent | Difloxacin | Sarafloxacin | Enrofloxacin | Ciprofloxacin | Enoxacin | Norfloxacin |
|---|---|---|---|---|---|---|
| a n.d., not detected; spiking level, 4 μg kg−1 each. | ||||||
| 0.1 M HCl | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 0.05 M orthophosporic acid | 0.6 | 17.8 | 5.4 | 16.9 | 22.7 | 19.5 |
| 5% HCOOH in acetonitrile | n.d. | 0.1 | n.d. | n.d. | n.d. | n.d. |
0.1 M phosphate buffer–acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 3.2) 1, v/v, pH 3.2) |
34.3 | 34.5 | 22.3 | 17.5 | 4.5 | 7.4 |
| 0.02 M phosphate buffer (pH 7.4) | 8.2 | 33.7 | 37.5 | 34.0 | 9.6 | 13.2 |
| 4% NH3·H2O in 50% Mg(NO3)2 solution | 64.6 ± 4.9 | 83.0 ± 5.2 | 78.2 ± 4.3 | 117 ± 6.6 | 101 ± 3.5 | 56.5 ± 6.7 |
| 0.1 M NaOH | 85.6 ± 3.3 | 89.6 ± 3.7 | 88.8 ± 1.4 | 87.9 ± 3.2 | 87.1 ± 6.0 | 89.6 ± 5.5 |
Thus, the concentration and volume of NaOH were further optimized. Firstly, the influence of the concentration of NaOH on the extraction efficiency was investigated in the concentration range of 0.01–0.5 M. The results reveal that the extraction efficiency of FQs increases with the increase of NaOH concentration. However, if the concentration of NaOH was too high, the recoveries of the other analytes decreased, especially up to 0.5 M, the recoveries of TCs, SQ and SCZ were significantly lowered. Secondly, the different volumes of NaOH solution were tested. The results show that the recoveries for FQs increased with the increase of the volume of NaOH solution. On the contrary, the recoveries for the other target analytes such as SAs and MLs decreased. The results are shown in Fig. 2. For a compromise, the 10 mL of 0.2 M NaOH was used for the following experiments.
 | ||
| Fig. 2 Influence of the concentration (a) and amount (b) of NaOH on the recoveries of 24 antimicrobials at the spiked 4 μg kg−1 each. The abbreviations are the same as Fig. 1. | ||
Finally, the ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2EDTA–McIlvaine buffer (5
Na2EDTA–McIlvaine buffer (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v, pH 4.0) system (M5) in combination with 0.2 M NaOH was selected to extract target analytes in soil samples.
5, v/v, pH 4.0) system (M5) in combination with 0.2 M NaOH was selected to extract target analytes in soil samples.
3.2. Cleanup
In complex environmental samples, for example sediments and soils, some matrix components can mask analytes in the chromatographic separation and in the final detection system.6 Therefore it is very necessary to choose the ideal SPE sorbents giving an acceptable recovery for all target compounds with different physicochemical properties. At present, the most commonly used SPE cartridges, which allow large sample volumes to be concentrated and purified in one step, are HLB,14 C18 (ref. 23) and MCX24 cartridges. In this study, three types of SPE (Bond Elut-C18 SPE C18, Oasis MCX and Oasis HLB) were evaluated. Each type of cartridge was processed at its optimal conditions. As shown in Fig. 3, recoveries less than 50% for most of the target analytes were obtained with both C18 and MCX cartridges, especially for SAs (below 10%). However, the HLB cartridge achieved the best recoveries (75–104%) for all analytes except for valnemulin (67%). So the HLB cartridge was chosen as the optimized SPE cartridge.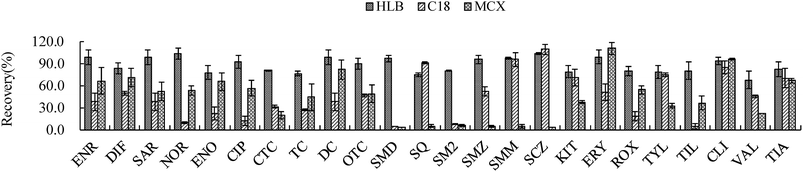 | ||
| Fig. 3 Influence of the different types of SPE columns on extraction efficiency for 24 antimicrobials at the spiked 4 μg kg−1 each. The abbreviations are the same as Fig. 1. | ||
3.3. Optimization of LC-MS/MS conditions
The electronic spray ionization-tandem mass spectrometer offers a high sensitivity and improved selectivity through multiple reactions monitoring acquisition to detect antimicrobials in real samples. The optimization of MS parameters for each compound was performed by direct infusion of pure reference standards (1 mg L−1) into the MS/MS compartment at 10 μL min−1 by a syringe pump (Harvard Apparatus, Holliston, MA). In the positive ion mode, the protonated molecules [M + H]+ were observed for all compounds on their full-scan mass spectra. These ions were selected as precursor ions to further produce product ions, and the corresponding parameters including declustering potential and collision energy in MRM mode were optimized. The results are listed in Table 1. For each analyte, two ion transitions were monitored; the first transition corresponding to the highest abundance was used for quantification and the second one for confirmation. Ion logarithms were selected in accordance with the 2002/657/EC requirements (IPs ≥ 4).25The chromatographic separation of the target compounds was performed using HPLC. The Zorbax SB-Aq column, which was proved to be superior to other chromatographic columns in our laboratory,26 was used for LC separation of the twenty-four analytes. In brief, acetonitrile was selected as eluent A and 0.1% formic acid in Milli-Q water was selected as eluent B. The linear gradient program was referred to the gradient program previously reported in Section 2.4.
3.4. Validation of the analytical method
 | ||
| Fig. 4 Typical MRM chromatograms obtained from the blank soil extracts (a) and blank soil extracts spiked at 4 μg kg−1 each (b). The abbreviations are the same as Fig. 1. | ||
| Group | Analyte | Linearity (r) | LOD (μg kg−1) | LOQ (μg kg−1) | Intra-day recovery, (%, n = 6) | Intra-day RSD, (%, n = 6) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 μg kg−1 | 4 μg kg−1 | 20 μg kg−1 | 1 μg kg−1 | 4 μg kg−1 | 20 μg kg−1 | |||||
| a LOD, limit of detection; LOQ, limit of quantification; SD, standard deviation; RSD, relative standard deviation; ME, matrix effect. | ||||||||||
| FQs | Difluoxacin | 0.9979 | 0.1 | 1.5 | 62.4 | 61.8 | 63.5 | 12 | 11 | 8.0 |
| Sarafloxacin | 0.9955 | 0.1 | 1.5 | 61.6 | 74.5 | 88.0 | 8.6 | 9.2 | 7.1 | |
| Enrofloxacin | 0.9938 | 0.05 | 0.4 | 65.2 | 70.6 | 68.3 | 6.0 | 7.4 | 5.5 | |
| Ciprofloxacin | 0.9981 | 0.2 | 0.5 | 61.7 | 77.5 | 78.9 | 9.5 | 9.0 | 7.2 | |
| Enoxacin | 0.9965 | 0.1 | 0.5 | 59.2 | 63.5 | 63.4 | 11 | 10 | 8.5 | |
| Norfloxacin | 0.9968 | 0.1 | 0.5 | 57.9 | 66.5 | 70.5 | 12 | 11 | 7.6 | |
| TCs | Chlortetracycline | 0.9961 | 0.2 | 1.0 | 60.0 | 60.8 | 66.7 | 13 | 8.3 | 6.7 |
| Oxytetracycline | 0.9974 | 0.2 | 1.0 | 70.4 | 68.0 | 71.4 | 14 | 12 | 4.4 | |
| Doxycycline | 0.9952 | 0.2 | 1.0 | 65.2 | 66.5 | 71.0 | 14 | 13 | 5.3 | |
| Tetracycline | 0.9978 | 0.5 | 1.5 | 53.8 | 60.1 | 67.4 | 9.5 | 3.4 | 8.0 | |
| SAs | Sulfaquinoxaline | 0.9948 | 0.3 | 1.0 | 60.5 | 63.7 | 75.0 | 12 | 9.0 | 6.6 |
| Sulfaclozine | 0.9972 | 1.0 | 2.0 | 55.4 | 68.1 | 60.0 | 10 | 5.9 | 7.0 | |
| Sulfamethoxydiazine | 0.9954 | 0.2 | 1.0 | 64.4 | 63.8 | 72.9 | 3.8 | 2.8 | 3.0 | |
| Sulfamonomethoxine | 0.9980 | 0.2 | 1.0 | 60.0 | 73.9 | 86.0 | 5.0 | 3.9 | 2.7 | |
| Sulfadimidine | 0.9959 | 0.5 | 1.0 | 60.8 | 61.9 | 63.4 | 5.4 | 6.7 | 6.0 | |
| Sulfamethoxazole | 0.9985 | 0.5 | 1.0 | 65.5 | 72.0 | 71.9 | 6.4 | 5.3 | 3.8 | |
| MLs | Tylosin | 0.9958 | 0.05 | 0.2 | 72.3 | 90.0 | 83.3 | 6.8 | 3.4 | 2.7 |
| Roxithromycin | 0.9988 | 0.05 | 0.2 | 83.0 | 79.8 | 80.6 | 4.9 | 5.0 | 5.0 | |
| Kitasamycin | 0.9970 | 1.0 | 2.5 | 79.5 | 75.0 | 79.8 | 6.2 | 3.5 | 2.4 | |
| Erythromycin | 0.9974 | 2.0 | 5.0 | 95.8 | 96.3 | 107 | 10 | 5.5 | 4.7 | |
| Tilmicosin | 0.9984 | 0.04 | 0.1 | 85.7 | 84.8 | 70.4 | 9.5 | 6.7 | 5.3 | |
| LAs | Clindamycin | 0.9968 | 0.01 | 0.04 | 80.6 | 84.0 | 93.3 | 8.0 | 4.4 | 3.0 |
| PMs | Valnemulin | 0.9974 | 0.05 | 0.3 | 60.3 | 61.2 | 61.5 | 8.1 | 7.8 | 6.5 |
| Tiamulin | 0.9956 | 0.05 | 0.2 | 70.8 | 78.5 | 75.0 | 6.7 | 6.0 | 3.3 | |
| Group | Analyte | Inter-day recovery, (%, n = 18) | Inter-day RSD, (%, n = 18) | ME (±SD) (%, n = 3) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 μg kg−1 | 4 μg kg−1 | 20 μg kg−1 | 1 μg kg−1 | 4 μg kg−1 | 20 μg kg−1 | |||
| FQs | Difluoxacin | 61.3 | 61.7 | 63.6 | 14 | 12 | 12 | 75.2 ± 7.9 |
| Sarafloxacin | 61.9 | 75.3 | 87.8 | 8.8 | 8.7 | 10 | 62.6 ± 4.9 | |
| Enrofloxacin | 64.8 | 69.9 | 66.0 | 6.1 | 7.4 | 13 | 73.4 ± 13 | |
| Ciprofloxacin | 62.9 | 79.9 | 76.4 | 9.5 | 8.8 | 7.8 | 69.0 ± 7.2 | |
| Enoxacin | 58.8 | 62.2 | 62.9 | 12 | 15 | 10 | 68.1 ± 1.5 | |
| Norfloxacin | 56.5 | 65.1 | 70.7 | 10 | 9.5 | 6.7 | 74.9 ± 10 | |
| TCs | Chlortetracycline | 59.9 | 58.2 | 65.2 | 11 | 8.8 | 7.3 | 71.3 ± 11 |
| Oxytetracycline | 71.5 | 68.3 | 71.6 | 14 | 10 | 3.9 | 86.9 ± 4.8 | |
| Doxycycline | 64.5 | 65.4 | 70.4 | 12 | 11 | 4.4 | 76.1 ± 2.0 | |
| Tetracycline | 53.2 | 58.6 | 66.0 | 10 | 2.9 | 8.8 | 68.6 ± 4.2 | |
| SAs | Sulfaquinoxaline | 60.9 | 59.4 | 75.1 | 12 | 10 | 5.6 | 56.8 ± 3.5 |
| Sulfaclozine | 54.4 | 67.0 | 60.2 | 9.3 | 5.4 | 8.2 | 78.0 ± 2.4 | |
| Sulfamethoxydiazine | 62.6 | 64.5 | 73.6 | 4.8 | 4.0 | 3.2 | 81.2 ± 6.7 | |
| Sulfamonomethoxine | 60.0 | 74.8 | 84.7 | 6.5 | 5.6 | 6.3 | 84.6 ± 4.8 | |
| Sulfadimidine | 58.9 | 61.1 | 64.3 | 7.9 | 9.4 | 5.1 | 62.3 ± 4.8 | |
| Sulfamethoxazole | 64.0 | 69.2 | 70.8 | 8.3 | 8.1 | 4.2 | 82.1 ± 2.2 | |
| MLs | Tylosin | 71.8 | 89.4 | 79.4 | 6.7 | 2.6 | 6.3 | 90.8 ± 3.0 |
| Roxithromycin | 82.4 | 81.0 | 79.3 | 4.9 | 4.9 | 5.2 | 93.4 ± 6.0 | |
| Kitasamycin | 75.9 | 75.6 | 77.0 | 5.5 | 3.5 | 5.7 | 90.4 ± 2.3 | |
| Erythromycin | 98.6 | 98.9 | 104 | 13 | 7.3 | 10 | 83.1 ± 2.7 | |
| Tilmicosin | 86.9 | 85.8 | 69.1 | 9.6 | 7.0 | 6.5 | 80.9 ± 5.7 | |
| LAs | Clindamycin | 81.5 | 85.5 | 92.9 | 9.3 | 5.7 | 3.3 | 97.3 ± 3.4 |
| PMs | Valnemulin | 58.5 | 60.9 | 61.7 | 8.8 | 8.6 | 10 | 80.8 ± 6.1 |
| Tiamulin | 72.6 | 77.3 | 74.1 | 3.7 | 7.2 | 2.9 | 79.9 ± 2.1 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the LOQ was defined as a S/N of 10
1 and the LOQ was defined as a S/N of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The results showed that clindamycin was higher sensitivity (0.01 μg kg−1 LOD) in the optimized LC-MS/MS conditions. The LODs of all target compounds ranged from 0.01 μg kg−1 to 2.0 μg kg−1 and the LOQs ranged from 0.04 to 5.0 μg kg−1 (Table 3). The developed method is sensitive enough for the determination of trace antimicrobials in soil samples.
1. The results showed that clindamycin was higher sensitivity (0.01 μg kg−1 LOD) in the optimized LC-MS/MS conditions. The LODs of all target compounds ranged from 0.01 μg kg−1 to 2.0 μg kg−1 and the LOQs ranged from 0.04 to 5.0 μg kg−1 (Table 3). The developed method is sensitive enough for the determination of trace antimicrobials in soil samples.3.5. Matrix effects
Matrix effects were evaluated at the concentrations of 20 μg kg−1. The matrix effects for each compound in soil from piggeries are summarized Table 3. Most antimicrobials experienced weak matrix suppression. There was matrix suppression at moderate intensity level (62.6–76.1%) for FQs and TCs except oxytetracycline (86.9%) and obvious matrix suppression for sulfaquinoxaline (56.8%). Although spiking appropriate internal standards and isotope dilution technique would eliminate for the matrix effects, large varieties of target compounds and the cost of isotope internal standard make this unfeasible. Therefore, this research adopted the matrix matching standard curve method to further compensate for matrix effects.3.6. Method application
A liquid chromatography-tandem mass spectrometric method based on the ESI multiple reaction monitoring mode for multiresidue analysis of 24 antimicrobials in soil was developed. Firstly, samples were extracted with acetonitrile–McIlvaine buffer system and 0.2 M sodium hydroxide solution, and then purified by solid phase extraction cartridge. Chromatographic separation was carried out on the Zorbax SB-Aq column using acetonitrile–0.1% formic acid as mobile phase with gradient program. For evaluating the applicability and performance of the proposed method, 100 soils samples collected from different sources (35 piggeries, 25 vegetable fields, 20 orchards and 20 living quarters) were examined. None of the target compounds was detected in the samples collected from the living quarters. However, other soil samples were found to be contaminated with at least four antimicrobials. The TCs were dominated antimicrobials detected in soil samples, especially the soils from piggeries with maximum level of 163.6 μg kg−1 chlortetracycline, followed by FQs (0.7–40.7 μg kg−1). Four analytes (kitasamycin, tiamulin, doxycycline and tilmicosin) were detected in the orchard soils at concentrations ranging from 1.5 μg kg−1 to 5.9 μg kg−1. Eight analytes (tiamulin, chlortetracycline, oxytetracycline, tetracycline, doxycycline, tilmicosin, enrofloxacin and sulfamonomethoxine) were found at concentrations ranging from 0.5 μg kg−1 to 18.3 μg kg−1 and ciprofloxacin and norfloxacin at levels of the quantification limits in the vegetable fields. The findings obtained in this study indicate that animal manure can cause veterinary pharmaceuticals contamination of agricultural soil. Some antimicrobials detected at relatively high concentrations in soil may be inferred that the animals were long-term administration and the pharmaceuticals were excreted through animal body as parent compounds.4. Conclusions
In this study a robust, sensitive and selective method has been developed and validated for the determination of 24 pharmaceuticals in soil matrices. The method has enabled accurate multiresidue determination of the target analytes in soil at μg kg−1 levels. The acceptable absolute recoveries were above 60% for most of the target compounds. This methodology was successfully applied to four different sources of soils including piggeries, vegetable fields, orchards and living quarters. Several commonly used antimicrobials such as chlortetracycline, enrofloxacin and tilmicosin were detected at different concentration levels. Even though some antimicrobials are detected at relatively low concentrations, there are high risks of their potential harms to human health.Acknowledgements
The authors thank the financial support by the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT13063) and the Joint Project of National Natural Science Foundation of China and Natural Science Foundation of Guangdong Province, China (U0631006) for this work.References
- V. Andreu, P. Vazquez-Roig, C. Blasco and Y. Picó, Anal. Bioanal. Chem., 2009, 394, 1329–1339 CrossRef CAS PubMed.
- W. L. Shelver, H. Hakk, G. L. Larsen, T. M. DeSutter and F. X. M. Casey, J. Chromatogr. A, 2010, 1217, 1273–1282 CrossRef CAS PubMed.
- M. P. Schlüsener, M. Spiteller and K. Bester, J. Chromatogr. A, 2003, 1003, 21–28 CrossRef.
- A. K. Sarmah, M. T. Meyer and A. B. A. Boxall, Chemosphere, 2006, 65, 725–759 CrossRef CAS PubMed.
- M. Salvia, E. Vulliet, L. Wiest, R. Baudot and C. Cren-Olivé, J. Chromatogr. A, 2012, 1245, 122–133 CrossRef CAS PubMed.
- E. Pérez-Carrera, M. Hansen, V. M. León, E. Björklund, K. A. Krogh, B. Halling-Sørensen and E. González-Mazo, Anal. Bioanal. Chem., 2010, 398, 1173–1184 CrossRef PubMed.
- W. Witte, Int. J. Antimicrob. Agents, 2000, 14, 321–325 CrossRef CAS.
- J. L. Zhou, K. Maskaoui and A. Lufadeju, Anal. Chim. Acta, 2012, 731, 32–39 CrossRef CAS PubMed.
- M. McDonald, C. Mannion and P. Rafter, J. Chromatogr. A, 2009, 1216, 8110–8116 CrossRef CAS PubMed.
- R. P. Lopes, D. V. Augusti, F. A. Santos, E. A. Vargas and R. Augusti, Anal. Methods, 2013, 5, 5121–5127 RSC.
- L. Zhou, G. Ying, S. Liu, J. Zhao, F. Chen, R. Zhang, F. Peng and Q. Zhang, J. Chromatogr. A, 2012, 1244, 123–138 CrossRef CAS PubMed.
- S. O'Connor and D. S. Aga, TrAC, Trends Anal. Chem., 2007, 26, 456–465 CrossRef PubMed.
- A. M. Jacobsen, B. Halling-Sørensen, F. Ingerslev and S. Honoré Hansen, J. Chromatogr. A, 2004, 1038, 157–170 CrossRef CAS PubMed.
- Y. B. Ho, M. P. Zakaria, P. A. Latif and N. Saari, J. Chromatogr. A, 2012, 1262, 160–168 CrossRef CAS PubMed.
- S. Morales-Muñoz, J. L. Luque-García and M. D. Luque De Castro, J. Chromatogr. A, 2004, 1059, 25–31 CrossRef PubMed.
- Y. Yamini, M. Asghari-Khiavi and N. Bahramifar, Talanta, 2002, 58, 1003–1010 CrossRef CAS.
- B. K. Matuszewski, M. L. Constanzer and C. M. Chavez-Eng, Anal. Chem., 2003, 75, 3019–3030 CrossRef CAS.
- N. Furusawa, J. AOAC Int., 1999, 82, 770–772 CAS.
- P. Kay, P. A. Blackwell and A. B. A. Boxall, Chemosphere, 2005, 60, 497–507 CrossRef CAS PubMed.
- M. M. Aguilera-Luiz, J. L. M. Vidal, R. Romero-González and A. G. Frenich, J. Chromatogr. A, 2008, 1205, 10–16 CrossRef CAS PubMed.
- B. Delepine, D. Hurtaud-Pessel and P. Sanders, Analyst, 1998, 123, 2743–2747 RSC.
- E. Turiel, A. Martín-Esteban and J. L. Tadeo, Anal. Chim. Acta, 2006, 562, 30–35 CrossRef CAS PubMed.
- D. N. Heller, M. A. Ngoh, D. Donoghue, L. Podhorniak, H. Righter and M. H. Thomas, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2002, 774, 39–52 CrossRef CAS.
- B. Kasprzyk-Hordern, R. M. Dinsdale and A. J. Guwy, J. Chromatogr. A, 2007, 1161, 132–145 CrossRef CAS PubMed.
- Commission of the European Communities, Off. J. Eur. Communities: Inf. Not., 2002, L221, 8–36 Search PubMed.
- F. Hu, K. Bian, Y. Liu, Y. Su, T. Zhou, X. Song and L. He, J. Chromatogr. A, 2014, 1368, 52–63 CrossRef CAS PubMed.
Footnote |
| † The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |

