Three-dimensional TiO2/CeO2 nanowire composite for efficient formaldehyde oxidation at low temperature†
Abstract
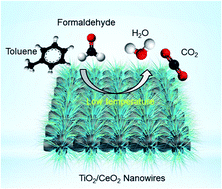
We developed a low-cost and high-performance TiO2/CeO2 nanowire-based catalyst for efficient catalytic volatile organic compound oxidation at low temperature. The TiO2/CeO2 nanowires yield a remarkable HCHO conversion efficiency of 60.2% at a low temperature of 60 °C and have excellent catalytic stability as well as good activity for toluene oxidation.

 Please wait while we load your content...
Please wait while we load your content...