Self-assembly of large-scale P3HT patterns by confined evaporation in the capillary tube†
Abstract
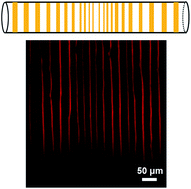
A facile and robust route to fabricate large area patterns of poly(3-hexylthiophene) (P3HT) was developed by controlled evaporative self-assembly (CESA) technique within a confined space (capillary tubes). Properties of P3HT, solvents effects and inner surface properties of the capillary tube were systematically investigated. The results showed that the patterns could be controlled to evolve from dot deposits and stripes with fingering instabilities, to highly regular, nearly perfect stripes. The orientation of P3HT within an individual stripe was characterized through confocal polarized Raman spectroscopy at molecular level, which revealed that the backbone chains of P3HT were parallel to the contact line. This simple method therefore provides a universal approach to control the morphology of patterns and chain orientation of functional polymers simultaneously.

 Please wait while we load your content...
Please wait while we load your content...