Stereoselective total synthesis of ent-hyptenolide†
Gembali Manikanta,
Galla Raju and
Palakodety Radha Krishna*
Organic & Biomolecular Chemistry Division, CSIR-Indian Institute of Chemical Technology, D-211, Discovery Laboratory, Hyderabad-500007, India. E-mail: prkgenius@iict.res.in; Fax: +91-40-27160387
First published on 22nd December 2014
Abstract
A stereoselective total synthesis of ent-hyptenolide is reported involving asymmetric allylation, Horner–Wadsworth–Emmons olefination, stereoselective anti reduction and RCM as the key steps.
Introduction
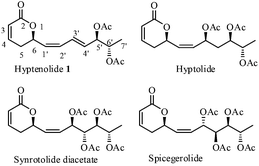
Hyptenolide (1, Fig. 1), a new α-pyrone was recently isolated from the aerial parts of Hyptis macrostachys Benth.1 The structure of 1 was deciphered by extensive NMR techniques and CD spectral data. The intriguing structural features of 1 include a unique combination of a trans olefin (C3′/C4′) in conjugation with a cis olefin (C1′/C2′) and the diene connected to the α-pyranone motif at C6. The distal end of the diene fragment is endowed with an anti-diacetoxy propane moiety. Compound 1 showed a selective spasmolytic effect in guinea pig trachea and ileum.1 In continuation of our interest in the synthesis of 5,6-dihydropyran-2-one2 containing natural products, the hitherto unreported structural features of 1 coupled with its impressive bio-active profile encouraged us to undertake its total synthesis.Results and discussion
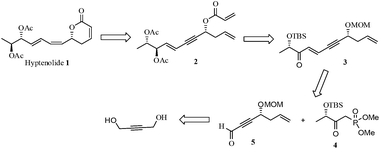
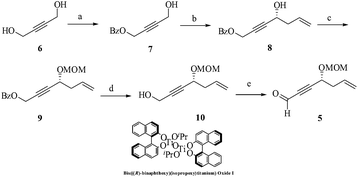
The envisaged retrosynthetic analysis of compound 1 is depicted in Scheme 1. Accordingly, 1 could be obtained from diacetoxy acryloyl derivative 2 on RCM. Compound 2 in turn could be derived from enyne 3 upon few chemical transformations such as silyl deprotection, selective reduction, acetylation, MOM-deprotection followed by acryloylation. Further, compound 3 maybe assembled using the HWE-olefination reaction between chiral ynal 5 and chiral phosphonate 4. Ynal 5 is conveniently drawn from commercially available 1,4-butyne diol involving Keck allylation to garner the stereogenic carbon and other sequential transformations like deprotection–oxidation reaction set. While phosphonate 4 itself was prepared from ethyl L-lactate by a reported procedure.3Accordingly, the synthesis began from the commercially available 1,4-butyne diol 6 (Scheme 2). Diol 6 on selective protection as its monobenzoate4 under conventional reaction conditions followed by oxidation (IBX conditions) afforded the corresponding aldehyde which on Keck allylation5 (R-BINOL/Ti(OiPr4)/TiCl4/Ag2O/allyltributyltin/CH2Cl2/−15 °C) provided the chiral propargylic alcohol 8 (75%). The absolute stereochemistry of the newly created stereogenic center was established using literature analogy and assigned as ‘R’.6 Next, protection of the secondary hydroxyl group as its MOM-ether (MOM-Cl/DIPEA/CH2Cl2/0 °C to rt) furnished compound 9 (88%). Subsequent hydrolysis (K2CO3/MeOH/0 °C–rt) of the benzoate 9 released the primary hydroxyl group to afford 10 (80%) in order to facilitate its oxidation (IBX/DMSO/CH2Cl2/0 °C to rt) and thus generate the crucial ynal fragment 5 (90%).
 | ||
| Scheme 2 Reagents and conditions: (a) ref. 4; (b) IBX, DMSO, CH2Cl2, 0 °C–rt, 90%, (ii) I, allyltributyltin (1.1 equiv.), CH2Cl2, −15 °C, 36 h (75%); (c) MOM-Cl, DIPEA, dry CH2Cl2, 0 °C–rt, 4 h, 88%; (d)K2CO3, CH3OH, 0 °C–rt, 80%; (e) IBX, DMSO, CH2Cl2, 0 °C–rt, 90%. | ||
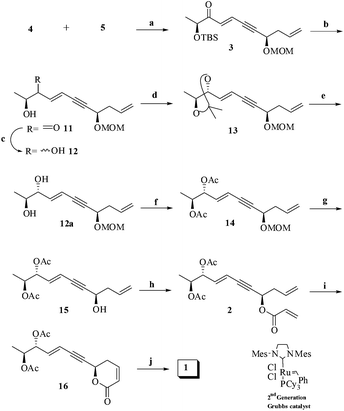
Another synthon, chiral phosphonate 4 was accessed using a reported procedure.3 Having the phosphonate 4 in hand, Horner–Wadsworth–Emmons olefination reaction was performed between phosphonate 4 and ynal 5 (Scheme 3) to result in enyne 3 (60%) as the separable E-isomer (>85%) as the major isomer. The E-geometry was ascertained from the 1H NMR spectrum wherein the newly formed olefinic protons resonated at δ 6.98 ppm as a doublet (J = 15.8 Hz, 1H) and another at δ 6.78 ppm as a dd (J = 1.8, 15.8 Hz, 1H). The other terminal olefinic protons appeared at their expected shifts. Next, we envisaged an anti-reduction of the accompanying keto group in enyne 3 would lead us to the total carbon framework with rightly positioned stereogenic centers. Firstly, desilylation of 3 (PPTS/MeOH/0 °C–rt) was effected to give the hydroxy ketone derivative 11 (78%). However, reduction7 (ZnBH4/THF/0 °C) of 11 offered an chromatographically inseparable diastereomeric mixture 12 (89% combined yield) in favor of the anti-isomer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, anti
1, anti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) syn) as the major compound. The diastereomeric ratio of 12 was determined from the 1H NMR spectrum by measuring the integration of the separable protons. For instance, while the terminal methyl protons of the minor isomer resonated at δ 1.19 ppm as a doublet (J = 6.4 Hz, 0.75H), the same protons for the major isomer resonated at δ 1.14 ppm as doublet (J = 6.4 Hz, 3H). Also, one of the olefinic protons for the minor isomer resonated at δ 6.19 ppm as a dd (J = 6.13, 15.8 Hz, 0.25H) while the corresponding proton for the major isomer resonated at δ 6.11 ppm as a dd (J = 6.1, 15.8 Hz, 1H). The relative stereochemistry of the major isomer was initially assigned as anti based on literature precedence.3,7 Thankfully, the diastereomeric mixture 12 could be chromatographically separated on its conversion to acetonide 13 (2,2 DMP/PPTS/CH2Cl2/0 °C to rt, 73%). As envisioned and to continue with the synthesis, optically pure anti-isomer (12a) was necessary. Hence, deprotection of the acetonide group was carried out under acidic conditions (60% aq. AcOH) to afford optically pure diol 12a (87%) that was diacetylated (Ac2O/Et3N/DMAP/CH2Cl2/0 °C to rt) to 14 (90%). In order to conclusively establish the anti-stereochemistry of the diol 12a, we conducted few more experiments. For example, keto compound 3 was subjected to Luche reduction8 followed by TBS-deprotection to afford syn-diol exclusively in 92% yield which was acetylated to afford 14a (90%) under conventional conditions. Likewise, the minor isomer obtained during the chromatographic separation of 13 was also converted into its diacetate after few transformations such as acetonide group deprotection followed by acetylation. Next, the comparative 1H NMR study of the thus obtained diacetates was made which showed a complete match, thus unequivocally establishing their syn diol relationship. Having ascertained the relatively stereochemistry of minor isomer, a comparison of 1H NMR data of 14 and 14a was also taken up (Fig. 2). Herein the two spectra displayed differences, notably the allylic proton H5′ in anti-isomer resonated downfield (δ 5.37 ppm) than its syn counter part (δ 5.33 ppm) in accordance with the literature report.9 Thus, the major compound 14 was conclusively proved as the anti-isomer drawing inference from all the above observations and its stereogenic carbon C5′ was assigned as ‘R’. Additionally, the absolute stereochemistry of the newly created stereogenic center was confirmed later through the synthesis.
syn) as the major compound. The diastereomeric ratio of 12 was determined from the 1H NMR spectrum by measuring the integration of the separable protons. For instance, while the terminal methyl protons of the minor isomer resonated at δ 1.19 ppm as a doublet (J = 6.4 Hz, 0.75H), the same protons for the major isomer resonated at δ 1.14 ppm as doublet (J = 6.4 Hz, 3H). Also, one of the olefinic protons for the minor isomer resonated at δ 6.19 ppm as a dd (J = 6.13, 15.8 Hz, 0.25H) while the corresponding proton for the major isomer resonated at δ 6.11 ppm as a dd (J = 6.1, 15.8 Hz, 1H). The relative stereochemistry of the major isomer was initially assigned as anti based on literature precedence.3,7 Thankfully, the diastereomeric mixture 12 could be chromatographically separated on its conversion to acetonide 13 (2,2 DMP/PPTS/CH2Cl2/0 °C to rt, 73%). As envisioned and to continue with the synthesis, optically pure anti-isomer (12a) was necessary. Hence, deprotection of the acetonide group was carried out under acidic conditions (60% aq. AcOH) to afford optically pure diol 12a (87%) that was diacetylated (Ac2O/Et3N/DMAP/CH2Cl2/0 °C to rt) to 14 (90%). In order to conclusively establish the anti-stereochemistry of the diol 12a, we conducted few more experiments. For example, keto compound 3 was subjected to Luche reduction8 followed by TBS-deprotection to afford syn-diol exclusively in 92% yield which was acetylated to afford 14a (90%) under conventional conditions. Likewise, the minor isomer obtained during the chromatographic separation of 13 was also converted into its diacetate after few transformations such as acetonide group deprotection followed by acetylation. Next, the comparative 1H NMR study of the thus obtained diacetates was made which showed a complete match, thus unequivocally establishing their syn diol relationship. Having ascertained the relatively stereochemistry of minor isomer, a comparison of 1H NMR data of 14 and 14a was also taken up (Fig. 2). Herein the two spectra displayed differences, notably the allylic proton H5′ in anti-isomer resonated downfield (δ 5.37 ppm) than its syn counter part (δ 5.33 ppm) in accordance with the literature report.9 Thus, the major compound 14 was conclusively proved as the anti-isomer drawing inference from all the above observations and its stereogenic carbon C5′ was assigned as ‘R’. Additionally, the absolute stereochemistry of the newly created stereogenic center was confirmed later through the synthesis.
Furthermore, diacetate 14 on deprotection of MOM group under Lewis acid conditions (TiCl4/CH2Cl2/0 °C) provided the homoallyl alcohol derivative 15 (80%). Subsequent acryloylation of 15 (acryloyl chloride/Et3N/DMAP/CH2Cl2/0 °C) afforded 2 (82%) which on Grubbs' catalyst10 assisted RCM (G-II/CH2Cl2/rt) resulted in the ring-closed product 16 (65%). Finally, partial hydrogenation of 16 under Lindlar's conditions gave the target compound 1 (90%).
All the spectral data of synthetic 1 matched1,11 with the reported data excepting the specific rotation {synthetic 1: [α]20D = −45.8 (c 0.2, CHCl3); natural 1 (ref. 1): [α]20D = +45.0 (c 0.001, CHCl3)}, which showed an opposite sign of rotation implying the synthesis of an enantiomer.
Conclusion
In summary, we accomplished the total synthesis of ent-hyptenolide in an overall yield of 3.9% from 7 using asymmetric allylation, Horner–Wadsworth–Emmons olefination, stereoselective anti reduction and RCM as the key steps.Experimental section
Reactions were carried out under N2 in dry solvents. All reactions were monitored by tlc and silica-coated plates were visualized by exposure to ultraviolet light and/or α-naphthol charring. Organic solutions were dried (Na2SO4) and concentrated below 40 °C under reduced pressure in a Büchi rotary evaporator. All column chromatographic (CC) separations were performed using silica gel (SiO2; 60–120 mesh) with EtOAc and hexane as eluents. Tlc was performed on Merck 60 F254 silica gel plates. Yields refer to chromatographically and spectroscopically (1H and 13C-NMR) homogeneous material. Air-sensitive reagents were transferred by syringe and double-ended needle. Optical rotations were measured on Anton Paar mcp-200 polarimeter and 1H NMR and 13C NMR spectra were recorded on Avance 300 or Avance 500 MHz nuclear magnetic resonance spectrometers. Chemical shifts were reported as (δ) in parts per million (ppm) with respect to internal TMS. Coupling constants J values are given in Hz. High-resolution mass spectra (HRMS) were obtained using either a TOF or a double focusing spectrometer.(R)-4-Hydroxyhept-6-en-2-yn-1-yl benzoate (8)
To an ice-cooled solution of IBX (9.94 g, 35.52 mmol) in DMSO (8.39 mL, 107.56 mmol) and CH2Cl2 (45 mL), was added a solution of alcohol 7 (4.5 g, 23.68 mmol) in CH2Cl2 (10 mL). The mixture was stirred at room temperature for 2 h and then filtered through a Celite pad and washed with CH2Cl2 (3 × 30 mL). The organic filtrate was sequentially washed with H2O, brine and later dried (Na2SO4) and concentrated in vacuo. The crude aldehyde (4.0 g, 90%, viscous liquid) was immediately subjected to the next reaction without further purification.To a stirred solution of TiCl4 (1 M solution in CH2Cl2, 2.1 mL, 2.1 mmol) in dry CH2Cl2 (40 mL) was added Ti(OiPr)4 (1.82 mL, 6.40 mmol) at 0 °C under N2. The solution was allowed to warm to room temperature. After 1 h, (R)-binaphthol (2.43 g, 8.49 mmol) was added at room temperature and the solution was stirred for 3 h. The mixture was cooled to 0 °C, and treated with silver(I) oxide (0.98 g, 4.25 mmol). The reaction mixture was allowed to warm to room temperature, and stirred there for 5 h under exclusion of direct light to furnish chiral bis Ti(IV) oxide (R,R)-I was treated with aldehyde (4.0 g, 21.27 mmol) and allyltributyltin (7.17 mL, 21.66 mmol) at −15 °C. The whole mixture was warmed to 0 °C and allowed to stir for 36 h. The reaction mixture was quenched with saturated NaHCO3, and extracted with CH2Cl2 (3 × 50 mL). The organic extracts were washed with brine, dried (Na2SO4), concentrated, and the residue was purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 3
n-hexane, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) to give the allylated product 8 (3.63 g, 75%) as yellow liquid.
7) to give the allylated product 8 (3.63 g, 75%) as yellow liquid.
[α]20D = −2.44 (c 1.4, CHCl3);1H NMR (500 MHz, CDCl3): δ 8.07 (dd, J = 1.3, 8.3 Hz, 2H), 7.58 (m, 1H), 7.48–7.43 (m, 2H), 5.88 (m, 1H), 5.23–5.17 (m, 2H), 4.96 (d, J = 1.6 Hz, 2H), 4.49 (t, J = 6.1 Hz, 1H), 2.52–2.48 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 165.8, 133.2, 132.7, 129.7, 129.4, 128.3, 119.1, 87.1, 79.2, 61.5, 52.7, 41.8; HRMS: m/z calcd for C14H18O3N [M + NH4]+: 248.1281; found: 248.1273.
(R)-4-(Methoxymethoxy)hept-6-en-2-yn-1-yl benzoate (9)
To compound 8 (3.5 g, 15.21 mmol) in dry CH2Cl2 (25 mL) at 0 °C, were successively added DIPEA (7.8 mL, 60.46 mmol), catalytic DMAP and MOMCl (1.82 mL, 22.75 mmol). The mixture was stirred for 4 h at room temperature, then the reaction was quenched by adding water (20 mL) and the mixture was extracted with CH2Cl2 (3 × 30 mL). The organic extracts were washed with brine, dried (Na2SO4) and the solvent was evaporated under reduced pressure. The crude material was purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 2
n-hexane, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford product 9 (3.66 g, 88%) as an colorless liquid.
8) to afford product 9 (3.66 g, 88%) as an colorless liquid.
[α]20D = + 71.0 (c 1.2, CHCl3);1H NMR (300 MHz, CDCl3): δ 8.06 (d, J = 7.1 Hz, 2H), 7.58 (t, J = 7.3 Hz, 1H), 7.45 (t, J = 7.7 Hz, 2H), 5.89 (m, 1H), 5.23–5.08 (m, 2H), 5.0–4.90 (m, 3H), 4.61 (d, J = 6.7 Hz, 1H), 4.43 (t, J = 6.4 Hz, 1H), 3.38 (s, 3H), 2.52 (t, J = 6.6 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 165.7, 133.2, 133.2, 129.7, 129.5, 128.3, 118.0, 94.1 85.0, 79.9, 65.1, 55.6, 52.6, 39.8; HRMS: m/z calcd for C16H22O4N [M + NH4]+: 292.1543; found: 292.1535.
(R)-4-(Methoxymethoxy)hept-6-en-2-yn-1-ol (10)
To stirred solution of 9 (3.2 g, 11.11 mmol) in MeOH (35 mL) was added potassium carbonate solid (2.3 g, 16.66 mmol) at 0 °C and allowed it to stir at room temperature. After stirring for 3 h, solvent MeOH was removed under reduced pressure. The crude residue was washed with water (3 × 20 mL) and extracted with EtOAc (2 × 50 mL). The organic layer was dried (Na2SO4), concentrated and purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 3.5
n-hexane, 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5) to obtain alcohol 10 (1.56 g, 80%) as a colorless liquid.
6.5) to obtain alcohol 10 (1.56 g, 80%) as a colorless liquid.
[α]20D = +90.0 (c 4.45, CHCl3); 1H NMR (300 MHz, CDCl3): δ 5.88 (m, 1H), 5.23–5.09 (m, 2H), 4.94 (d, J = 6.7 Hz, 1H), 4.61 (d, J = 6.7 Hz, 1H), 4.42 (t, J = 6.4 Hz, 1H), 4.30 (d, J = 0.94 Hz, 2H), 3.38 (s, 3H), 2.50 (t, J = 6.6 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 133.2, 117.9, 93.9, 84.3, 83.5, 65.2, 55.6, 50.7, 39.9; m/z: C9H18O3N (M + NH4)+: 188.
(5R,11S,E)-5-Allyl-11,13,13,14,14-pentamethyl-2,4,12-trioxa-13-silapentadec-8-en-6-yn-10-one (3)
To an ice-cooled solution of IBX (3.45 g, 12.32 mmol) in DMSO (3.21 mL, 41.15 mmol) and CH2Cl2 (20 mL), was added a solution of alcohol 10 (1.40 g, 8.23 mmol) in CH2Cl2 (5 mL). The mixture was stirred at room temperature for 2 h and then filtered through a Celite pad and washed with CH2Cl2 (3 × 30 mL). The combined organic filtrates were washed with H2O (2 × 10 mL) and brine (10 mL), dried (Na2SO4), and concentrated in vacuo. The crude aldehyde 5 (1.24 g, 90%) was immediately subjected to the next reaction without further purification.Cs2CO3 (2.37 g, 7.29 mmol) was added to a solution of 4 (1.13 g, 3.64 mmol) in MeCN (10 mL), and was stirred for 45 min at room temperature. The reaction mixture was cooled to −15 °C and a solution of the aldehyde 5 (1.24 g, 7.29 mmol) in MeCN (10 mL) was added drop wise and stirred for 45 min at the same temperature. After completion of the reaction, it was cautiously quenched by addition of saturated citric acid (10 mL), poured into water (30 mL), and extracted with diethyl ether (3 × 50 mL). Combined organic layers were washed with brine (2 × 20 mL) and dried (Na2SO4). Evaporation of solvent gave the crude residue, which was purified by column chromatography using (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 1
n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as eluent to furnish 3 (E-isomer, 1.54 g, 60%) as a light yellow oil.
9) as eluent to furnish 3 (E-isomer, 1.54 g, 60%) as a light yellow oil.
[α]20D = +17.7 (c 1.1, CHCl3);1H NMR (500 MHz, CDCl3): δ 6.98 (d, J = 15.8 Hz, 1H), 6.78 (dd, J = 1.6, 16.0 Hz, 1H), 5.88 (m, 1H), 5.22–5.13 (m, 2H), 4.91 (d, J = 6.8 Hz, 1H), 4.63 (d, J = 6.8 Hz, 1H), 4.55 (td, J = 1.6, 6.5 Hz, 1H), 4.26 (q, J = 6.8 Hz, 1H), 3.39 (s, 3H), 2.60–2.50 (m, 2H), 1.30 (d, J = 6.7 Hz, 3H), 0.91 (s, 9H), 0.07 (s, 3H), 0.06 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 200.5, 132.9, 132.5, 123.5, 118.2, 97.1, 94.3, 83.9,74.2, 65.7, 55.7, 39.7, 25.7, 20.9, 18.1, −4.8, −5.0; HRMS: m/z calcd for C19H33O4Si [M + H]+: 353.2142; found: 353.2181.
(2S,8R,E)-2-Hydroxy-8-(methoxymethoxy)undeca-4,10-dien-6-yn-3-one (11)
To a stirred solution of 3 (1.2 g, 3.40 mmol) in MeOH (20 mL) was added PPTS (1.71 g, 6.81 mmol) at room temperature and it was stirred for 12 h. After completion of the reaction (monitored by tlc), MeOH was evaporated under vacuum and the reaction mixture was diluted with CH2Cl2 (20 mL). Solid NaHCO3 was added to the reaction mixture and was stirred for further 15 min. The reaction mixture was then filtered through a short pad of Celite and was washed with CH2Cl2 (3 × 20 mL) and the solvent was evaporated under reduced pressure. The crude product was purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 3
n-hexane, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) to obtain alcohol 11 (0.632 g, 78%) as a colorless oil.
7) to obtain alcohol 11 (0.632 g, 78%) as a colorless oil.
[α]20D = +64.2 (c 0.3, CHCl3);1H NMR (500 MHz, CDCl3): δ 6.86 (dd, J = 1.8, 15.8 Hz, 1H), 6.63 (d, J = 15.8 Hz, 1H), 5.87 (m, 1H), 5.22–5.14 (m, 2H), 4.91 (d, J = 6.8 Hz, 1H), 4.63 (d, J = 6.8 Hz, 1H), 4.56 (td, J = 1.6, 6.4 Hz, 1H), 4.42 (q, J = 7.0 Hz, 1H), 3.39 (s, 3H), 2.60–2.50 (m, 2H), 1.40 (d, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 199.8, 132.8, 132.2, 124.7, 118.4, 98.8, 94.3, 83.1, 71.8, 65.6, 55.7, 39.6, 19.9; HRMS: m/z calcd for C13H18O4Na [M + Na]+: 261.1097; found: 261.1106.
(4R,5S)-4-((R,E)-5-(Methoxymethoxy)octa-1,7-dien-3-yn-1-yl)-2,2,5-trimethyl-1,3 dioxolane (13)
To a solution of zinc borohydride (4.2 M solution in THF, 0.56 mL, 2.39 mmol) was added drop wise a solution of ketone 11 (0.570 g, 2.39 mmol) in dry THF (10 mL), at 0 °C and under N2. It was stirred for 30 min. the mixture was quenched with sat. aq. NH4Cl and extracted with ethyl acetate. The combined organic extracts were washed with brine, dried (Na2SO4) and the solvent was evaporated under reduced pressure. The crude product was purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 1
n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gave the 4
1) gave the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture of diol 12 (0.511 g, 89%) as colorless liquid.
1 diastereomeric mixture of diol 12 (0.511 g, 89%) as colorless liquid.
To a solution of diol 12 (0.460 g, 1.91 mmol) in dry CH2Cl2 (5 mL), 2,2-dimethoxy propane (0.47 mL, 4.5 mmol) and PPTS (0.048 g, 0.19 mmol) were added at 0 °C. The mixture was stirred at room temperature for 3 h. Next, solid NaHCO3 was added to the reaction mixture and was stirred for further 15 min and filtered. Removal of solvent and purification by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 2
n-hexane, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) gave the required anti-isomer 13 (0.394 g, 73%) as a colorless liquid.
8) gave the required anti-isomer 13 (0.394 g, 73%) as a colorless liquid.
[α]20D = +55.2 (c 0.8, CHCl3);1H NMR (300 MHz, CDCl3): δ 6.05 (dd, J = 7.1, 15.8 Hz, 1H), 5.90 (m, 1H), 5.79 (dt, J = 1.1, 15.8 Hz, 1H), 5.23–5.10 (m, 2H), 4.94 (d, J = 6.7 Hz, 1H), 4.61 (d, J = 6.7 Hz, 1H), 4.55–4.45 (m, 2H), 4.35 (quint, J = 6.4 Hz, 1H), 3.38 (s, 3H), 2.52 (t, J = 6.7 Hz, 2H), 1.49 (s, 3H), 1.36 (s, 3H), 1.16 (d, J = 6.4 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 139.5, 133.4, 117.8, 111.9, 108.3, 94.0, 88.3, 83.6, 78.8, 74.1, 65.6, 55.6, 40.0, 28.0, 25.4, 16.0; m/z: C16H24O4Na[M + Na]+: 303.
(2S,3R,8R,E)-8-(Methoxymethoxy)undeca-4,10-dien-6-yne-2,3-diol (12a)
To a solution of 13 (0.3 g, 1.07 mmol) was added 3 mL of aqueous 60% acetic acid at room temperature and it was stirred for 6 h. After completion of the reaction, it was cautiously quenched by addition of solid NaHCO3, filtered and washed with EtOAc (3 × 10 mL), dried (Na2SO4) and the solvent was evaporated under reduced pressure. The crude product was purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 1
n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain diol 12a (0.223 g, 87% yield) as a colorless liquid.
1) to obtain diol 12a (0.223 g, 87% yield) as a colorless liquid.
[α]20D = +88.9 (c 1.2, CHCl3); 1H NMR (300 MHz, CDCl3): δ 6.16 (dd, J = 6.2, 16.0 Hz, 1H), 5.96–5.76 (m, 2H), 5.22–5.10 (m, 2H), 4.94 (d, J = 6.9 Hz, 1H), 4.60 (d, J = 6.8 Hz, 1H), 4.49 (dt, J = 1.5, 6.4 Hz, 1H), 4.14 (m, 1H), 3.88 (m, 1H), 3.38 (s, 3H), 2.52 (t, J = 6.6 Hz, 2H), 1.14 (d, J = 6.42 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 141.1, 133.3, 117.9, 111.6, 94.0, 88.3, 83.6,75.4, 70.0, 65.6, 55.6, 40.0, 17.4; HRMS: m/z calcd for C13H20O4Na [M + Na]+: 263.1253; found: 263.1262.
(2S,3R,8R,E)-8-(Methoxymethoxy)undeca-4,10-dien-6-yne-2,3-diyl diacetate (14)
To a solution of diol 12a (0.170 g, 0.708 mmol) in dry CH2Cl2 (6 mL), pyridine (0.17 mL, 2.15 mmol), Ac2O (0.2 mL, 1.96 mmol) and DMAP (cat.) were added slowly. The mixture was then stirred for 2 h at room temperature. After completion of the reaction (tlc monitoring), the mixture was extracted with EtOAc (2 × 10 mL), and the combined organic layer was washed with brine (5 mL), dried (Na2SO4), concentrated and purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 2
n-hexane, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to furnish 14 (0.206 g, 90%) as a viscous liquid.
8) to furnish 14 (0.206 g, 90%) as a viscous liquid.
[α]20D = +36.3 (c 1.9, CHCl3);1H NMR (500 MHz, CDCl3): δ 6.05 (dd, J = 7.0, 16.0 Hz, 1H), 5.89 (m, 1H), 5.79 (d, J = 16.0 Hz, 1H), 5.37 (dd, J = 3.5, 7.0 Hz, 1H), 5.20–5.12 (m, 2H), 5.06 (dq, J = 3.6, 6.7, Hz, 1H), 4.92 (d, J = 6.8 Hz, 1H), 4.60 (d, J = 6.86 Hz, 1H), 4.49 (t, J = 6.4 Hz, 1H), 3.38 (s, 3H), 2.55–2.48 (m, 2H), 2.08 (s, 3H), 2.05 (s, 3H), 1.20 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 170.1, 169.6, 136.3, 133.2, 117.9, 113.9, 94.0, 89.3, 82.9, 74.4, 70.1, 65.4, 55.5, 39.9, 21.0, 20.8, 15.0; HRMS: m/z calcd for C17H24O6Na [M + Na]+: 347.1465; found: 347.1475.
(2S,3R,8R,E)-8-Hydroxyundeca-4,10-dien-6-yne-2,3-diyl diacetate (15)
To a stirred solution of compound 14 (0.160 g, 0.493 mmol) in dry CH2Cl2 (20 mL) was added TiCl4 (1 M solution in CH2Cl2, 0.396 mL, 0.396 mmol) at 0 °C and the reaction mixture was stirred at the same temperature for 1 h. The reaction mixture was quenched with solid NaHCO3 (0.050 g) and filtered. The solvent was removed under reduced pressure. The residue was purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 4
n-hexane, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) to afford 15 (0.110 g, 80%) as a colorless liquid.
6) to afford 15 (0.110 g, 80%) as a colorless liquid.
[α]20D = −5.8 (c 2.1, CHCl3);1H NMR (500 MHz, CDCl3): δ 6.05 (dd, J = 7.0, 16.0 Hz, 1H), 5.88 (m, 1H), 5.79 (dt, J = 1.5, 16.0 Hz, 1H), 5.37 (ddd, J = 1.2, 3.6, 7.0 Hz, 1H), 5.22 (m, 1H), 5.19 (t, J = 1.0 Hz, 1H), 5.07 (dq, J = 3.5, 6.5 Hz, 1H), 4.54 (t, J = 5.4 Hz, 1H), 2.52–2.47 (m, 2H), 2.08 (s, 3H), 2.05 (s, 3H), 1.20 (d, J = 6.7 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 170.2, 169.7, 136.4, 132.7, 119.1, 113.9, 91.4, 82.3, 74.5, 70.2, 61.8, 41.9, 21.0, 20.9, 15.1; HRMS: m/z calcd for C15H24O5N [M + NH4]+: 298.1649; found: 298.1658.
(2S,3R,8R,E)-8-(Acryloyloxy)undeca-4,10-dien-6-yne-2,3-diyl diacetate (2)
Acryloyl chloride (0.027 mL, 0.306 mmol) was added drop wise under N2 to a stirred solution of 15 (0.080 g, 0.285 mmol), and Et3N (0.047 mL, 0.46 mmol) in CH2Cl2 (5 mL). The mixture was stirred at 0 °C for 1 h. After completion, the mixture was poured into brine (5 mL), and extracted with CH2Cl2 (2 × 10 mL), dried (Na2SO4) and concentrated. The crude product was purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 2
n-hexane, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford the corresponding acrylic ester 2 (0.078 g, 82%) as a yellow color oil.
8) to afford the corresponding acrylic ester 2 (0.078 g, 82%) as a yellow color oil.
[α]20D = +0.17 (c 1.4, CHCl3); 1H NMR (500 MHz, CDCl3): δ 6.45 (d, J = 17.3 Hz, 1H), 6.18–6.04 (m, 2H), 5.90–5.75 (m, 3H), 5.59 (t, J = 6.4 Hz, 1H), 5.37 (dd, J = 3.5, 6.8 Hz, 1H), 5.22–5.13 (m, 2H), 5.06 (m, 1H), 2.59 (t, J = 6.5 Hz, 2H), 2.08 (s, 3H), 2.05 (s, 3H), 1.20 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 170.2, 169.7, 164.9, 137.0, 131.9, 131.5, 127.9, 118.8, 113.5, 87.8, 82.9, 74.4, 70.1, 63.6, 39.0, 21.0, 20.8, 15.0; HRMS: m/z calcd for C18H22O6Na [M + Na]+: 357.1308; found: 357.1320.
(2S,3R,E)-7-((R)-6-Oxo-3,6-dihydro-2H-pyran-2-yl)hept-4-en-6-yne-2,3-diyl diacetate (16)
To a solution of compound 2 (0.040 g, 0.119 mmol) in CH2Cl2 (40 mL) was added Grubbs' second generation catalyst (0.01 g, 0.0117 mmol) at room temperature. The reaction mixture was stirred overnight at room temperature. The solvent was evaporated and the crude product was purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 4
n-hexane, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) to give lactone 16 (0.023 g, 65%) as a pale-yellow oil.
6) to give lactone 16 (0.023 g, 65%) as a pale-yellow oil.
[α]20D = −0.36 (c 0.17, CHCl3); 1H NMR (300 MHz, CDCl3): δ 6.89 (m, 1H), 6.18–6.05 (m, 2H), 5.77 (d, J = 16.0 Hz, 1H), 5.40–5.28 (m, 2H), 5.07 (dq, J = 3.5, 6.4 Hz, 1H), 2.73–2.65 (m, 2H), 2.09 (s, 3H), 2.06 (s, 3H), 1.20 (d, J = 6.6 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 170.2, 169.7, 162.4, 143.8, 138.0, 121.5, 112.9, 86.3, 83.6, 74.3, 70.1, 67.3, 30.0, 21.0, 20.9, 15.1; HRMS: m/z calcd for C16H18O6Na [M + Na]+: 329.0995; found: 329.1009.
(2S,3R,4E,6Z)-7-((R)-6-Oxo-3,6-dihydro-2H-pyran-2-yl)hepta-4,6-diene-2,3-diyl diacetate {ent-hyptenolide 1}
To a solution of 16 (0.009 g, 0.0294 mmol) in EtOAc (2 mL), one drop of quinoline and Lindlar's catalyst (Pd/CaCO3, catalytic amount) were added and the mixture was stirred at room temperature under H2 for 20 min. After completion of the reaction, the mixture was filtered and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane, 1
n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 1 (0.008 g, 90%) as an oil.
1) to afford 1 (0.008 g, 90%) as an oil.
[α]20D = −45.8 (c 0.2, CHCl3);1H NMR (500 MHz, CDCl3): δ 6.91 (ddd, J = 2.8, 5.4, 9.9 Hz, 1H), 6.51 (tdd, J = 1.0, 11.2, 15.1 Hz, 1H), 6.17 (br. t, J = 11.3 Hz, 1H), 6.08 (ddd, J = 1.2, 2.2, 9.7 Hz, 1H), 5.76 (dd, J = 7.1, 15.2 Hz, 1H), 5.64 (dd, J = 8.6, 10.8 Hz, 1H), 5.45 (ddd, J = 1.0, 3.3, 7.1 Hz, 1H), 5.35 (m, 1H), 5.07 (dq, J = 3.5, 6.5 Hz, 1H), 2.48–2.32 (m, 2H), 2.10 (s, 3H), 2.05 (s, 3H), 1.21 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 170.3, 169.9, 163.6, 144.5, 131.1, 130.3, 128.5, 128.1, 121.6, 74.5, 73.7, 70.4, 29.7, 21.1, 21.0, 14.9; HRMS: m/z calcd for C16H20O6Na [M + Na]+: 331.1152; found: 331.1161.
Acknowledgements
Two of the authors (GM and GR) thank the UGC and CSIR, New Delhi respectively for financial support in the form of fellowships. Authors thank the CSIR, India for financial support as part of XII Five Year Plan program under title TREAT (BSC-0116).References
- V. C. de O Costa, J. F. Tavares, A. B. Silva, M. C. Duarte, M. de. F. Agra, J. M. Barbosa-Filho, I. L. L. de Souza, B. A. da Silva and M. S. Silva, Phytochem. Lett., 2014, 8, 32–37 CrossRef PubMed.
- (a) P. Radha Krishna and V. V. Ramana Reddy, Tetrahedron Lett., 2005, 46, 3905–3907 CrossRef PubMed; (b) P. Radha Krishna and R. Srinivas, Tetrahedron Lett., 2007, 48, 2013–2015 CrossRef PubMed; (c) P. Radha Krishna and R. Srinivas, Tetrahedron: Asymmetry, 2007, 18, 2197–2200 CrossRef PubMed; (d) P. Radha Krishna and P. Srinivas Reddy, Tetrahedron, 2007, 63, 3995–3999 CrossRef PubMed; (e) P. Radha Krishna and K. Lopinti, Beilstein J. Org. Chem., 2009, 5, 2–6 Search PubMed; (f) P. Radha Krishna and P. Srinivas, Tetrahedron Lett., 2010, 51, 2295–2296 CrossRef CAS PubMed; (g) P. Radha Krishna, R. Nomula and R. Kunde, Synthesis, 2014, 46, 307–312 CrossRef PubMed; (h) G. Dayaker and P. Radha Krishna, Helv. Chim. Acta, 2014, 97, 868–880 CrossRef CAS.
- G. Shapiro, D. Buechler and S. Hennet, Tetrahedron Lett., 1990, 31, 5733–5736 CrossRef CAS.
- M. M. Fraser and R. A. Raphael, J. Chem. Soc., 1955, 4280–4283 RSC.
- G. E. Keck, K. H. Tarbet and L. S. Geraci, J. Am. Chem. Soc., 1993, 115, 8467–8468 CrossRef CAS.
- A. de Fatima, L. K. Kohn, M. A. Antônio, J. E. de Carvalhob and R. A. Pillia, Bioorg. Med. Chem., 2004, 12, 5437–5442 CrossRef CAS PubMed.
- T. Nakata, T. Tanaka and T. Oishi, Tetrahedron Lett., 1983, 24, 2653–2656 CrossRef CAS.
- K. R. Prasad and P. Gutala, Tetrahedron, 2012, 68, 7489–7493 CrossRef CAS PubMed.
- J. M. Contelles, E. de Opazo and N. Arroyo, Tetrahedron, 2001, 57, 4729–4739 CrossRef.
- (a) A. K. Chatterjee and R. H. Grubbs, Angew. Chem., Int. Ed., 2002, 41, 3171–3174 CrossRef CAS; (b) R. H. Grubbs, Tetrahedron, 2004, 60, 7117–7140 CrossRef CAS PubMed.
- For all the spectral data please see the ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13708f |
| This journal is © The Royal Society of Chemistry 2015 |