Antimony tin oxide porous layers improve the poly(3,4-ethylenedioxythiophene) counter electrode fabricated by vapor deposition for dye-sensitized solar cells†
Xiang Xiaa,
Wenyi Wub,
Jiahui Mab,
Tingting Liub,
Dehou Feia,
Xizhe Liu*a and
Chunxiao Gaob
aJilin Provincial Key Laboratory of Applied Atomic and Molecular Spectroscopy, Institute of Atomic and Molecular Physics, Jilin University, Changchun 130012, China. E-mail: liu_xizhe@jlu.edu.cn; Fax: +8643185168816; Tel: +8643185168817
bState Key Laboratory for Superhard Materials, Jilin University, Changchun 130012, China
First published on 27th January 2015
Abstract
In this paper, we develop high performance poly(3,4-ethylenedioxythiophene) (PEDOT) counter electrodes (CEs) by a vapor deposition method, which gives 6.09% in conversion efficiency for the dye-sensitized solar cell (DSSC) with a thin PEDOT film CE (16 nm in thickness). To increase the quantity of PEDOT deposited on the CEs, we insert an antimony tin oxide (ATO) porous conductive supporting layer to increase the internal deposition area. The conversion efficiency of the DSSC with this PEDOT/ATO composite CE is enhanced to 7.47%.
Dye-sensitized solar cells (DSSC) are kinds of low-cost thin film photovoltaic devices, which were first reported by Grätzel's group in 1991.1 They attract world-wide attention for their simple fabrication process and relatively high solar-to-electric conversion efficiency.2,3 The most commonly used counter electrode (CE) material in DSSC is platinum (Pt), because Pt has good conductivity and high electrocatalytic activity for the reduction process of redox couples.4 However, Pt is an expensive metal with low abundance, which motivates researchers to find alternative materials. Several Pt-free materials have been reported,5–15 such as carbon materials, sulfides and conducting polymers. In these materials, the conducting polymer is a promising candidate, which is due to its adjustable properties, good film-forming ability and high catalytic activity.
Poly(3,4-ethylenedioxythiophene) (PEDOT) is a widely used conducting polymer for CEs, and several effective PEDOT CEs have been reported.16–30 To further decrease the production cost of PEDOT CEs, researchers develop in situ polymerization method, which use low cost monomers instead of expensive polymers as the precursor material. The in situ chemical polymerization need to introduce metal ions (for example Fe3+), which will decrease the photoelectric performance of the dye-sensitized TiO2 electrode. The in situ electrochemical polymerization is an effective fabrication method, which does not introduce metal ions. This electrochemical process is highly sensitive with the electrode potential, and the fabrication of uniform PEDOT film will depend on the high conductivity of substrates. Recently, new in situ method of solid state polymerization was reported for fabricating PEDOT CEs, and this method was not limited by substrates.29,30 But in this method, monomers will exist in the PEDOT film because of the low polymerization degree, and the monomer also has the problem of sublimation in the polymerization process. These factors will influence the quality of PEDOT films. Chemical vapor deposition usually can give high quality films. Depending on the same polymerization reaction of solid state polymerization,31,32 vapor based in situ polymerization can also be performed. As the vapor deposition usually has low growth rate, we introduce a porous substrate with large internal area to increase the quantity of formed PEDOT. For the porous substrate, high conductivity is important for transporting electrons out of the electrode. Antimony tin oxide (ATO), a stable conductive oxide, can satisfy this requirement. In this study, we fabricate PEDOT coated porous ATO (PEDOT/ATO) electrodes by vapor deposition method for the Pt-free counter electrodes of DSSCs, and their photoelectric and electrochemical performances are studied.
Four kinds of CEs were assembled, which included Pt electrode, ATO porous electrode, PEDOT electrode and PEDOT/ATO composite electrode. Briefly, fluorine doped tin oxide (FTO) glasses were cleaned and ATO nanoparticle paste solution were deposited by spin coating. Then, the samples were sintered to get the ATO porous supporting layer. PEDOT electrodes and PEDOT/ATO composite electrodes were fabricated by vapor deposition method. A crystalline 2,5-dibromo-3,4-ethylenedioxythiophene (DBEDOT) was placed in a closed vessel with substrates together. PEDOT were deposited on two kinds of substrates, bare FTO and FTO with ATO porous film. The system was purged with nitrogen and pumped (about 1000 Pa) before placed in an oven at 70 °C for 3 h. Then, blue PEDOT film was produced on the substrates. Finally, the PEDOT film was treated with LiClO4 in acetonitrile solution. The CEs were assembled with the prepared TiO2 photoanodes in sandwich structure to get the solar cell devices.
Schematic illustration of vapor deposition process is shown in Scheme 1a. Energy Disperse Spectroscopy (EDS) measurement demonstrates the concentration of Sb element in the ATO nanoparticles is 8.7 wt% (Fig. S1†). In Scheme 1b, FTO glass and ATO coated FTO glass have same peak positions in the X-ray diffraction (XRD) patterns, which can be assigned to the tetragonal structure of SnO2. As no extra characteristic peak can be detected, the Sb atoms should be doped into the crystal lattice of SnO2. In Scheme 1c, the PEDOT deposited on FTO surface is light blue, but the PEDOT deposited on ATO layer is deep blue in color. In Scheme 1d, these two PEDOT films have similar position in the absorption band, which belongs to the absorption of PEDOT,31 but their absorption intensity has about twenty times in difference at the absorption band between 650 nm and 1100 nm. This difference indicates that the ATO porous layer can remarkable increase the quantity of deposited PEDOT on the CEs. The absorption band at about 790 nm can be assigned to the polaron and/or bipolaron band, which comes from the interaction between oxide and PEDOT. Previous study indicates this interaction can increase the photoelectric activity of the oxide/PEDOT system.33
Fig. 1a and b shows the morphology of the FTO glass substrates before and after coated with PEDOT through vapor deposition method. In Fig. 1a, the FTO layer is a polycrystalline film with average crystal size of about 100 nm, and the film has dense structure. Fig. 1b shows that PEDOT film tends to keep the morphology of the original FTO layer, and its thickness is 16 nm (XP-2Profiler, AMBIOS TECHNOLOGY). Fig. 1c and d shows the morphology of the ATO layer before and after coated with PEDOT through vapor deposition. As shown in Fig. 1c, the ATO layer is formed by nanoparticles with about 20 nm in size, and its thickness is about 190 nm (Fig. 1e). Its porous structure provides large internal area for the vapor deposition, which will increase the quantity of deposited PEDOT. This is in accordance with the relative large absorption of the PEDOT/ATO composite electrode in Scheme 1d. Fig. 1d shows that this composite electrode keeps the original porous structure of the ATO layer.
 | ||
| Fig. 1 SEM surface morphology images of FTO glass (a), PEDOT coated FTO glass (b), ATO porous layer (c), PEDOT coated ATO porous layer (d) and the cross section of ATO porous layer on FTO glass (e). | ||
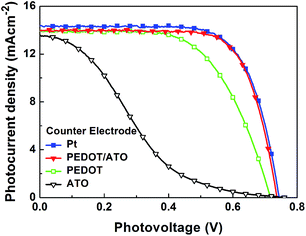
Fig. 2 shows the photocurrent density–photovoltage curves of DSSCs based on the four different CEs, and the parameters are summarized in Table 1. As shown in Table 1, device based on Pt CE exhibits the solar-to-electric conversion efficiency of 7.54%. Comparing with this reference device, device based on PEDOT/ATO composite CE can achieves equivalent performance (7.47%), while the PEDOT CE without ATO porous layer has a relatively low conversion efficiency of 6.09%. The devices based on these two kinds of PEDOT electrodes have similar open circuit photovoltage (Voc) and short circuit photocurrent density (Jsc), but they have a difference in fill factor (FF, 0.72 and 0.61, respectively). ATO porous CE gains lowest conversion efficiency of 2.07% with lowest FF of 0.20. These results indicate that the PEDOT/ATO composite structure is effective for assembling Pt-free CEs. Comparing with electrochemical polymerization and solid state polymerization,25,29,30 the limited quantity of PEDOT (16 nm in thickness) in the PEDOT CE without ATO layer can also give a reasonable performance, which implies the high quality of vapor deposited PEDOT film.
| Counter electrode | Voca (V) | Jscb (mA cm−2) | Fill factor | ηc % | Rsd (Ω) | Rcte (Ω) | Npf (Ω) |
|---|---|---|---|---|---|---|---|
| a Open circuit photovoltage.b Short circuit photocurrent density.c Solar-to-electric conversion efficiency.d The series resistance.e The charge transfer resistance at the electrode/electrolyte interface.f The Nernst diffusion impedance due to the diffusion of the redox ions through the counter electrode pores. | |||||||
| Pt | 0.743 | 14.4 | 0.71 | 7.54 | 19.68 | 3.99 | N.A. |
| PEDOT/ATO | 0.737 | 14.0 | 0.72 | 7.47 | 19.87 | 3.52 | 4.48 |
| PEDOT | 0.716 | 13.9 | 0.61 | 6.09 | 19.70 | 36.9 | 50.4 |
| ATO | 0.759 | 13.6 | 0.20 | 2.07 | 19.13 | 1.82 × 104 | N.A. |
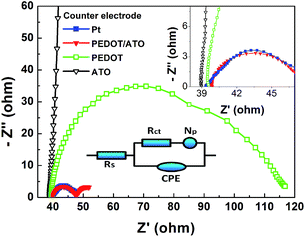
Electrochemical impedance spectra (EIS) of the dummy cells based on different CEs are given in Fig. 3, which is carried out to analyze the electrochemical characteristics of different counter electrodes. The dummy cells are composed of two identical electrodes and the same electrolyte used in devices. With the equivalent circuit model, we fit the impedance spectra and summarize the parameters in Table 1. In Fig. 3, the arches demonstrate the charge transfer process and diffusion process in the dummy cells. As previous report,28,34 the high-frequency feature is attributed to the charge transfer resistance (Rct) at the electrode/electrolyte interface. The decrease in this resistance, which is due to the increase in electrocatalytic activity, improves fill factor of DSSCs. As shown in Table 1, the Rct (3.52 Ω, 0.25 cm2) of PEDOT/ATO composite electrode is similar with the Rct (3.99 Ω, 0.25 cm2) of Pt electrode. It indicates these two electrodes have similar activity. Besides, the Rct of PEDOT/ATO composite electrode is much smaller than that (36.9 Ω, 0.25 cm2) of PEDOT electrode. It indicates the charge transfer process, which occurs on the surface of the PEDOT/ATO composite electrode, is much easier than that of the PEDOT electrode. Furthermore, Np represents the diffusion process of redox ions in the PEDOT films. The PEDOT/ATO composite electrode (4.48 Ω) has lower diffusion resistance than the PEDOT electrode (50.4 Ω). This implies that the channels of the porous structure in the PEDOT/ATO composite electrode may promote the transport of ions. The huge charge transfer resistance (1.82 × 104 Ω) of bare ATO electrode indicates that ATO has little electrochemical activity for the redox couples. The EIS analysis is also performed on the completed DSSC devices (Fig. S2†), which demonstrates the performance of electrodes in the working condition. The result is accordance with the analysis on the dummy cells.
Cyclic voltammograms of the CEs are measured using a three-electrode system to study catalytic activity for the tri-iodide ion reduction process. The result can be seen in Fig. 4. Two pairs of redox peaks are assigned to the following reaction.35
| 3I− − 2e ⇄ I3− | (a) |
| 2I3− − 2e ⇄ 3I2 | (b) |
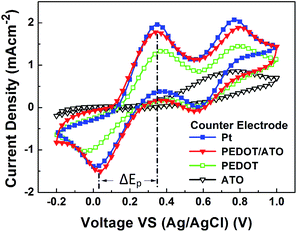 | ||
| Fig. 4 Cyclic voltammograms of Pt electrode, PEDOT/ATO composite electrode, PEDOT electrode, and ATO electrode. Scan range is from −0.2 V to 1.0 V, and the scan rate is 50 mV s−1. | ||
The first anodic peak corresponds to reaction (a). As shown in Fig. 4, the peak potential difference (ΔEp) for I3−/I− reaction on the PEDOT/ATO composite electrode is 0.315 V, while the ΔEp on the Pt electrode and PEDOT electrode are 0.340 V and 0.419 V. The lowest ΔEp means that the I3−/I− reaction on the PEDOT/ATO composite electrode performs most easily among the electrodes. No redox peaks are observed on the ATO electrode, which shows its low electrocatalytic activity in the voltage range. Besides, the anodic and cathodic peak current ratio (Ipa/Ipc) on the PEDOT/ATO composite electrode (1.18) is closer to 1 than the Ipa/Ipc (1.40) on the Pt electrode and the Ipa/Ipc (1.30) on the PEDOT electrode. This means the PEDOT/ATO composite electrode has the best reversibility among the four different CEs. Furthermore, with this ATO porous layer, the current density of reduction peak increases from 1.03 mA cm−2 to 1.51 mA cm−2, which indicates that the reduction reaction rate on PEDOT/ATO composite electrode is faster than that on the PEDOT electrode without ATO porous layer. This result is accordance with the increased PEDOT quantity of the PEDOT/ATO electrode in Scheme 1d and its highly porous structure in Fig. 1d. These improvements increase the contact area at the electrode/electrolyte interface and promote the electrochemical activity of the PEDOT/ATO electrode.
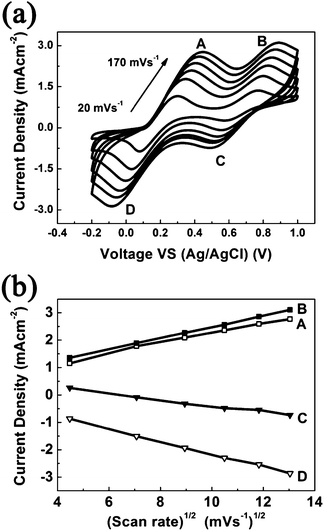
As shown in Fig. 5a, cyclic voltammograms of the I2/I− system on the PEDOT/ATO composite electrode are collected at different scan rates (20–170 mV s−1). The cathodic peak and anodic peak gradually move to the opposite direction with the increase of scan rates. Furthermore, Fig. 5b shows good linear relationship between the square root of scan rates and the redox peak current densities. It indicates the redox reaction on the PEDOT/ATO composite electrode is diffusion controlled, which is due to the transport of iodide species out of the PEDOT/ATO composite electrode surface.25,34,36
To further understand the role of the ATO supporting layer, the thickness effect of ATO films on the PEDOT/ATO composite electrodes is investigated. The surface morphology and cross-section of these ATO layers and composite electrodes are illustrated in Fig. S3.† Fig. 6 shows the photocurrent density–photovoltage curves of DSSCs based on these PEDOT/ATO composite electrodes, and the photovoltaic parameters are summarized in Table 2. The 200 nm ATO layer gives the best performance. Decreasing the thickness of ATO layers, the fill factor of the corresponding device is degraded from 0.71 to 0.67, which result in the decrease of the conversion efficiency (from 7.42% to 6.58%). A thin porous ATO supporting layer leads to low quantity of deposited PEDOT, then the electrode/electrolyte contact area will decrease and the electrochemical activity of the electrode will decrease. On the other hand, for a thick ATO layer of 500 nm, the conversion efficiency of the corresponding device decreases to 5.99%. The low performance comes from the increase of the ion diffusion distance in the pore channels of the electrode. The optimum thickness comes from the balance between electrode/electrolyte contact area and ion diffusion distance.
 | ||
| Fig. 6 Photocurrent density–photovoltage curves of DSSCs, which are fabricated with PEDOT/ATO composite electrodes based on different ATO layer thickness. | ||
As shown in Fig. 7, DSSC based on PEDOT/ATO composite electrode is measured continuously with 1 min interval to study the electrochemical stability. Its initial conversion efficiency is 7.27%, which increases to 7.42% after 10 times measurements. Compare with the spin-coating electrode (Fig. S5†), the PEDOT/ATO composite electrode demonstrates better performance on stability, which benefits from the efficient PEDOT/ATO architecture and the vapor deposition method.
Conclusions
In conclusion, we fabricated efficient PEDOT composite electrodes with ATO porous supporting layers by the vapor deposition method for dye-sensitized solar cells. Benefiting from the high quality of vapor deposited PEDOT layer, the device with PEDOT CE of only 16 nm in thickness gives a reasonable performance. By inserting a porous ATO layer, the quantity of vapor deposited PEDOT can be remarkable increased. As a consequence, the DSSC based on PEDOT/ATO composite electrode achieved a solar-to-electric conversion efficiency of 7.47%, which is equivalent to the classical Pt electrode and superior to the PEDOT electrode without ATO layer. Additionally, this design of catalytic electrodes would have potential applications in other electrochemical devices.Acknowledgements
This work was partially supported by the National Science Foundation of China (Grant nos 51273079, 91014004, 11374121), the National Basic Research Program of China (Grant no. 2011CB808204), the Fundamental Research Funds for Jilin University and Chunmiao Program of Jilin Province.Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef.
- C. Y. Chen, M. K. Wang, J. Y. Li, N. Pootrakulchote, L. Alibabaei, C. H. Ngoc-le, J. D. Decoppet, J. H. Tsai, C. Gratzel, C. G. Wu, S. M. Zakeeruddin and M. Gratzel, ACS Nano, 2009, 3, 3103 CrossRef CAS PubMed.
- H. J. Snaith, Adv. Funct. Mater., 2010, 20, 13 CrossRef CAS.
- X. Yin, Z. S. Xue and B. Liu, J. Power Sources, 2011, 196, 2422 CrossRef CAS PubMed.
- J. Zhang, F. Zhao, G. Tang and Y. Lin, J. Solid State Electrochem., 2013, 17, 2909 CrossRef CAS PubMed.
- S. N. Yun, H. H. Pu, J. H. Chen, A. Hagfeldt and T. L. Ma, ChemSusChem, 2014, 7, 442 CrossRef CAS PubMed.
- M. Wang, A. M. Anghel, B. Marsan, N.-L. C. Ha, N. Pootrakulchote, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2009, 131, 15976 CrossRef CAS PubMed.
- W. Liu, Y. Fang, P. Xu, Y. Lin, X. Yin, G. Tang and M. He, ACS Appl. Mater. Interfaces, 2014, 6, 16249 CAS.
- H. Sun, D. Qin, S. Huang, X. Guo, D. Li, Y. Luo and Q. B. Meng, Energy Environ. Sci., 2011, 4, 2630 CAS.
- H. Sun, Y. Luo, Y. Zhang, D. Li, Z. Yu, K. Li and Q. B. Meng, J. Phys. Chem. C, 2010, 114, 11673 CAS.
- Q. Zhang, Y. Liu, Y. Duan, N. Fu, Q. Liu, Y. Fang, Q. Sun and Y. Lin, RSC Adv., 2014, 4, 15091 RSC.
- P. W. Chen, C. P. Lee, L. Y. Chang, J. Chang, M. H. Yeh, L. Y. Lin, R. Vittal, J. J. Lin and K. C. Ho, RSC Adv., 2013, 3, 5871 RSC.
- R. K. Bhosale, S. A. Agarkar, I. Agrawal, R. A. Naphade and S. Ogale, RSC Adv., 2014, 4, 21989 RSC.
- S. N. Yun, A. Hagfeldtand and T. L. Ma, Adv. Mater., 2014, 26, 6210 CrossRef CAS PubMed.
- J. D. Roy-Mayhew, D. J. Bozym, C. Punckt and I. A. Aksay, ACS Nano, 2010, 4, 6203 CrossRef CAS PubMed.
- Y. Saito, T. Kitamura, Y. Wada and S. Yanagida, Chem. Lett., 2002, 10, 1060 CrossRef.
- Y. Saito, W. Kubo, T. Kitamura, Y. Wada and S. Yanagida, J. Photochem. Photobiol., A, 2004, 164, 153 CrossRef CAS PubMed.
- B. Fan, X. Mei, K. Sun and J. Ouyang, Appl. Phys. Lett., 2008, 93, 143103 CrossRef PubMed.
- H. Xu, X. Zhang, C. Zhang, Z. Liu, X. Zhou, S. Pang, X. Chen, S. Dong, Z. Zhang, L. Zhang, P. Han, X. Wang and G. Cui, ACS Appl. Mater. Interfaces, 2012, 4, 1087 CAS.
- J. M. Pringle, V. Armel and D. R. MacFarlane, Chem. Commun., 2010, 46, 5367 RSC.
- X. Z. Liu, W. Zhang, S. Uchida, L. Cai, B. Liu and S. Ramakrishna, Adv. Mater., 2010, 22, E150 CrossRef CAS PubMed.
- G. Yue, J. Wu, Y. Xiao, J. Lin and M. Huang, Electrochim. Acta, 2012, 67, 113 CrossRef CAS PubMed.
- K.-M. Lee, W.-H. Chiu, H.-Y. Wei, C.-W. Hu, V. Suryanarayanan, W.-F. Hsieh and K.-C. Ho, Thin Solid Films, 2010, 518, 1716 CrossRef CAS PubMed.
- W. Hong, Y. Xu, G. Lu, C. Li and G. Shi, Electrochem. Commun., 2008, 10, 1555 CrossRef CAS PubMed.
- J. Zhang, X. Li, W. Guo, T. Hreid, J. Hou, H. Su and Z. Yuan, Electrochim. Acta, 2011, 56, 3147 CrossRef CAS PubMed.
- S. Ahmad, J.-H. Yum, X. Zhang, M. Grätzel, H.-J. Butt and M. K. Nazeeruddin, J. Mater. Chem., 2010, 20, 1654 RSC.
- K. S. Lee, H. K. Lee, D. H. Wang, N.-G. Park, J. Y. Lee, O. O. Park and J. H. Park, Chem. Commun., 2010, 46, 4505 RSC.
- Z. Zhang, X. Zhang, H. Xu, Z. Liu, S. Pang, X. Zhou, S. Dong, X. Chen and G. Cui, ACS Appl. Mater. Interfaces, 2012, 4, 6242 CAS.
- L. Chen, J. Jin, X. Shu and J. Xia, J. Power Sources, 2014, 248, 1234 CrossRef CAS PubMed.
- X. Yin, F. Wu, N. Fu, J. Han, D. Chen, P. Xu, M. He and Y. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 8423 CAS.
- H. Meng, D. F. Perepichka, M. Bendikov, F. Wudl, G. Z. Pan, W. J. Yu, W. J. Dong and S. Brown, J. Am. Chem. Soc., 2003, 125, 15151 CrossRef CAS PubMed.
- H. Meng, D. F. Perepichka and F. Wudl, Angew. Chem., Int. Ed., 2003, 42, 658 CrossRef CAS PubMed.
- T. Abdiryim, A. Ali, R. Jamal, Y. Osman and Y. Zhang, Nanoscale Res. Lett., 2014, 9, 89 CrossRef PubMed.
- A. Hauch and A. Georg, Electrochim. Acta, 2001, 46, 3457 CrossRef CAS.
- A. I. Popov and D. H. Geske, J. Am. Chem. Soc., 1958, 80, 1340 CrossRef CAS.
- S. Biallozor and A. Kupniewska, Electrochem. Commun., 2000, 2, 480 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, cross-sectional SEM images of ATO film and Nyquist plots of the DSSCs. See DOI: 10.1039/c4ra13591a |
| This journal is © The Royal Society of Chemistry 2015 |