Anti-proliferative activity of the combination of salan Ti(IV) complexes with other organic and inorganic anticancer drugs against HT-29 and NCI-H1229 cells: synergism with cisplatin†
Nitzan Ganota,
Boris Redkob,
Gary Gellermanb and
Edit Y. Tshuva*a
aThe Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 91904, Israel. E-mail: edit.tshuva@mail.huji.ac.il; Fax: +972-2-6584282
bDepartment of Biological Chemistry, Ariel University of Samaria, Ariel 40700, Israel
First published on 12th December 2014
Abstract
Two diaminobis(phenolato) Ti(IV) complexes were separately combined with azatoxin, camptothecin or cisplatin and underwent in vitro cytotoxicity analysis toward HT-29 and NCI-H1229 cancer cell lines. The methylated salan derivative exhibited synergistic effects with cisplatin at varying ratios toward both lines, when the compounds were administered fresh and simultaneously, implying great medicinal potential.
The search for metal based anticancer drugs has evolved significantly during the past decades.1 Cisplatin, although highly effective towards particular tumour types, suffers from severe side effects and a narrow activity range and resistance development;2 thus, additional metal based drugs that may operate by different mechanisms are sought for. Titanium is a promising drug candidate; its complexes showed reduced toxicity and wide activity range.1a,3 In particular, the diaminobis(phenolato) “salan” Ti(IV) complexes developed in our group are highly effective anti-tumor agents, showing enhanced stability in water solutions, accompanied by high cytotoxicity towards various human and murine, drug-sensitive and drug-resistant cell lines, and negligible effect on primary murine cells.3b,4 L1Ti(OiPr)2 and L2Ti(OiPr)2 (Scheme 1) were identified as leading compounds among the salan family of Ti(IV) complexes in respect to their high hydrolytic stability and cytotoxicity,4a,b and are the subject of current intensive research.
Combination therapy of two or more drugs operating by different mechanisms is a common methodology to achieve efficient tumor-growth inhibition while reducing side effects.5 An additive effect of the administered drugs enables reducing the dose of each drug to achieve the desired effect, and consequently the toxicity of both drugs is reduced. In particular, a synergistic effect of the combined drugs, in which the drugs operate more effectively together than the sum of each individual drug, offers a substantial improvement in the drug efficiency.
Numerous combinations of drugs have been employed in cancer treatment.6 For instance, attempts to overcome the cell resistance phenomenon and the high toxicity of cisplatin involved combining it with various anti-cancer agents,7 including paclitaxel,7b,8 topoisomerase inhibitors,9 and others.2,7a,10 The behaviour of the combinations ranged from synergistic to antagonistic (or “sub-additive”5b), depending on the ratio of the drugs, the doses, the period of exposure to the cells, and the order of the administration.8–10 Platinum based compounds are often used in actual treatment as a combination with other drugs, such as paclitaxel for ovarian cancer.11
The derivatives of camptothecin (CPT; Scheme 2) are widely used as chemotherapeutic drugs to treat several cancer types, including ovarian cancer, small cell lung cancer, colorectal cancer and more.12 Unlike cisplatin, operating on the target bio-molecule DNA, the only known cellular target of camptothecin is topoisomerase I. Azatoxin (Scheme 2) is also a topoisomerase inhibitor, but unlike CPT, the target of azatoxin is topoisomerase II, and it also inhibits tubulin polymerization.13 Thus, analysis of combination therapy with such potent anticancer agents operating by varying mechanisms is beneficial for medicinal applications. Herein we present in vitro analysis of the cytotoxicity of combinations of salan Ti(IV) complexes with the known anti-cancer drug cisplatin, CPT and azatoxin, while evaluating the interactions between them using the isobolographic method.14 Different behaviours were detected, including highly synergistic ones of great potential in medicinal utility.
L1Ti(OiPr)2 and L2Ti(OiPr)2 (Scheme 1) were prepared as previously described from the ligand precursor and Ti(OiPr)4, and characterized by NMR.4b,f The anti-proliferative activity of the complexes was measured in vitro in combination with cisplatin, CPT, or azatoxin, in comparison to that of the compounds when administered alone (Fig. 1); the cells were treated with the investigated complex, known agent, or a combination of the two at different concentrations, and after a three days incubation period, analysis was carried out by the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay.15 The interaction between the combined agents was analysed by the isobolographic method (Fig. 2–5).14
Isobolographic analysis is a method to identify the nature of the interactions of two potential drugs. The isobologram is a 2-D graph, presenting the concentrations employed of the two potential drugs combined that together induce a single effect level, usually reducing the response by 50% of the maximal cell growth inhibition (IC50). Each axis represents a single drug concentrations (CA and CB), and thus the IC50 values of the drugs, when administrated alone, are plotted as axial points. The line connecting these points is the additive isobole, which represents the equation where the combination index (CI) is 1:
The two drugs are combined at fixed ratio and the IC50 of the mixture, namely, the concentration point for which 50% cell growth inhibition was achieved, is determined. The concentration of each drug at that IC50 concentration point is derived and plotted on the graph. Points located under the additive line (CI < 1) correspond to a synergistic behavior, those above the line (CI > 1) correspond to an antagonistic behavior, and those on the line (CI = 1) or within the error range represent an additive behaviour.
The ratio between the administrated agents, R, is defined as the concentration of the agent presented on the X axis (salan Ti(IV) complex) divided by that of the agent presented on the Y axis (cisplatin, azatoxin or CPT).
Combination of salan Ti(IV) complexes with different drugs
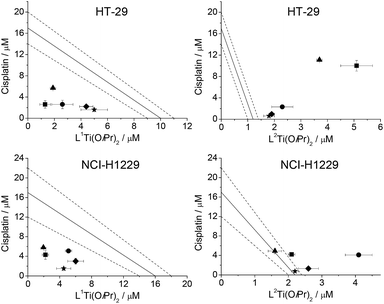
Cytotoxicity measurements of the combination of L1Ti(OiPr)2 and L2Ti(OiPr)2 with azatoxin, CPT, and cisplatin were performed on the relatively resistant, representative colon HT-29 cell line. The ratio between the administrated drugs, R, was constant for each combination. This value was chosen according to the ratio of their IC50 values when the two agents are administrated alone, to achieve similarly effective responses. The results are presented in Fig. 2.The combinations of both L1,2Ti(OiPr)2 with CPT showed an additive behaviour. This implies that there is no interaction between CPT and the Ti(IV) complexes, and every anti-cancer agent reacts as if the other is not present. However, the behaviours of the combinations with azatoxin and cisplatin depended on the salan complex. Whereas the combination of azatoxin or cisplatin with L2Ti(OiPr)2 demonstrated an antagonistic behaviour, that with L1Ti(OiPr)2 showed a synergistic effect. Such effects may point either to two different mechanisms of the combined drugs that relate to one another, or to an interaction of the two compounds that might be either beneficial or detrimental. Under the assumption that the mechanisms of action of the two complexes are similar, this marked difference in the behaviour was unexpected. One explanation might relate to the differences in steric bulk, as L2Ti(OiPr)2 is more sterically bulky than L1Ti(OiPr)2. Steric effects were previously reported to negatively influence the performance of salan Ti(IV) complexes due to reduced biological accessibility.4a,16 It is thus possible that the bulkier compound produces a larger product of interaction with the second drug with limited accessibility, or penetrates the cell more slowly itself thus affecting the schedule of its arrival at the target (see below).
Combination of salan Ti(IV) complexes with cisplatin at varying ratios
Elaborated analysis was performed on the combination of the salan Ti(IV) complexes with cisplatin, being the leading inorganic anti-cancer drug with defined mechanism of action2 and well-established potential utility of its combination with other drugs.7b,8–10 The anti-proliferative activity of the combinations was analysed against human colon HT-29 and non-small cell lung NCI-H1229 cancer cells. Different ratios of concentration of the administered drugs were employed; as the IC50 values of L1,2Ti(OiPr)2 and cisplatin are generally in the same order of magnitudes, R = 0.33, 0.5, 1, 2, and 3 were analysed. The results are presented in Fig. 3.The concentration ratio of the administered drugs did not have significant influence on the behaviours of the combination. The combination of L1Ti(OiPr)2 with cisplatin at all ratios showed synergistic behaviour against both cell lines analysed, whereas that of L2Ti(OiPr)2 showed a repeating antagonistic behaviour. Nevertheless, it is noticeable that for the L2Ti(OiPr)2–cisplatin combination administered to HT-29 cells, as the R ratio increased, i.e. the L2Ti(OiPr)2 concentration is higher than that of cisplatin, the behaviour became less antagonistic. Another interesting observation relates to the behaviour of the same combination when administered to NCI-H1229 cells line: at ratios for which one of the reagents is more dominant than the other, i.e. R = 0.33 and R = 3, there is less interference between the reagents and the behaviour becomes additive. These results may support the hypothesis that the behaviour of the combinations result from interaction between the two drugs, which is more effective in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.
1 ratio.
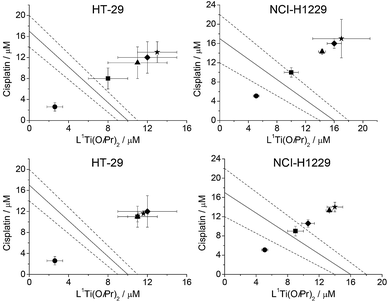
Combination of salan Ti(IV) complexes with cisplatin at varying administration schedules
As previous studies have indicated that the order of administration of combined drugs may affect the behaviour of the combination,8 the effect of administration scheduled was investigated. The compounds were added to the cells consequently with varying time intervals in between, while maintaining short enough intervals to prevent cell cycle processes from influencing the results. L1Ti(OiPr)2 was combined with cisplatin at ratio R = 1, which showed synergism when administered simultaneously, and the anti-proliferative activity was analysed against human colon HT-29 and non-small cell lung NCI-H1229 cancer cells. The results are summarized in Fig. 4.Regardless of the order of administration, both when the cells were treated first with L1Ti(OiPr)2 and then with cisplatin, and vice versa, a clear pattern was observed toward both cell lines: as the time interval between administration of the compounds increased, the behaviour became more antagonistic (Fig. 4).8 It is also noteworthy that similar results were obtained when the medium of the cells was replaced before the insertion of the second anticancer agent (see ESI†). It is thus clear that the synergistic behaviour is only possible when the cells are exposed to the two anticancer agents simultaneously. This may support the notion that the behaviour of the combination is a result of interaction of the two drugs prior to arrival at the cells, which is only possible when the drugs are administrated as a combined solution.
Suspecting that the source for the synergistic behaviour is a reaction between the salan complex L1Ti(OiPr)2 and cisplatin, in an additional experiment, the two drugs were allowed to pre-incubate together as an organic mixture of their administration solvents at 37 °C prior to addition to the cells, and the anti-proliferative activity of the combination was analysed against colon HT-29 and non-small cell lung NCI-H1229 cells (Fig. 5). Interestingly, the results indicated that the beneficial interaction between the drugs was lost, and the behaviour turned either additive or antagonistic after long pre-incubation periods. It is thus presumed that dissociation of the active species occurs during long sitting under non-inert conditions. This dissociation apparently results both from susceptibility of the salan Ti(IV) complex to hydrolysis and from the decreased activity of cisplatin after sitting in DMSO solution (see ESI†).17 Alternatively, any potential chemical product of the interaction between the drugs may also undergo dissociation to species of reduced activity.
Conclusions
In in vitro testing of two representative cell lines, a salan Ti(IV) complex exhibits synergism with the effective anti-cancer agent cisplatin, at various ratios of the administered drugs. This observation implies a significant therapeutic value of this and related titanium compounds, particularly due to their reduced toxicity relative to the platinum counterparts.3c,18 Nevertheless, the behaviour of the combination depends not only on the combined drug, but also on the particular derivative of the salan complex employed and order of administration. Whereas the combination with CPT showed an additive behaviour for both salan Ti(IV) complexes, that with azatoxin and cisplatin pointed to some interaction either between the drugs themselves or between their mechanisms of operations, which was constructive for one derivative but destructive for the other. Although the two salan Ti(IV) complexes operating by different mechanisms cannot be ruled out as an explanation to their different behaviours, an alternative explanation relates to their different chemical structure, affecting a potential chemical interaction with a combined drug or their biological accessibility. As the brominated derivative is bulkier and more stable, it is possible that it more slowly yields a bulkier product with reduced biological accessibility. Additionally, the combination of the methylated salan Ti(IV) derivative with cisplatin showed synergism only when both compounds were administered “fresh” and simultaneously, suggesting decomposition of the compounds or their product when not in the presence of cells. Nevertheless, such an administration is also favoured for future in vivo and clinical studies.To summarize, we found that most combinations analysed with L1Ti(OiPr)2 are synergistic or additive, both are medicinally valuable. Since anti-cancer chemotherapy widely relies on combinations of drugs to reduce the side effect of each drug, and due to the reduced toxicity Ti(IV) in general, further exploration of the salan family of Ti(IV) complexes is clearly of great medicinal value. Additionally, although the mechanism of the salan Ti(IV) complexes cannot be unequivocally determined at this point, their operation on DNA or as topoisomerases inhibitors cannot be ruled out.3g,19 Further mechanistic analysis of the operation of these compounds is thus also of the essence for further development of efficient compounds and combination therapy regimes, and is currently ongoing in our laboratory.4e,20
Acknowledgements
Funding was received from the European Council under the European Community's Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement (no. 239603), and from the Israel Science Foundation (grant no. 124/09).Notes and references
- (a) I. Ott and R. Gust, Arch. Pharm., 2007, 340, 117–126, DOI:10.1002/ardp.200600151; (b) S. H. van Rijt and P. J. Sadler, Drug Discovery Today, 2009, 14, 1089–1097, DOI:10.1016/j.drudis.2009.09.003; (c) M. A. Jakupec, M. Galanski, V. B. Arion, C. G. Hartinger and B. K. Keppler, Dalton Trans., 2008, 183–194, 10.1039/b712656p; (d) P. C. A. Bruijnincx and P. J. Sadler, Curr. Opin. Chem. Biol., 2008, 12, 197–206, DOI:10.1016/j.cbpa.2007.11.013; (e) B. Desoize, Anticancer Res., 2004, 24, 1529–1544 CAS; (f) M. Galanski, V. B. Arion, M. A. Jakupec and B. K. Keppler, Curr. Pharm. Des., 2003, 9, 2078–2089, DOI:10.2174/1381612033454180.
- (a) D. Wang and S. J. Lippard, Nat. Rev. Drug Discovery, 2005, 4, 307–320, DOI:10.1038/nrd1691; (b) L. Kelland, Nat. Rev. Cancer, 2007, 7, 573–584, DOI:10.1038/nrc2167.
- (a) P. M. Abeysinghe and M. M. Harding, Dalton Trans., 2007, 3474–3482, 10.1039/b707440a; (b) E. Y. Tshuva and J. A. Ashenhurst, Eur. J. Inorg. Chem., 2009, 2203–2218, DOI:10.1002/ejic.200900198; (c) E. Melendez, Crit. Rev. Oncol. Hematol., 2002, 42, 309–315, DOI:10.1016/s1040-8428(01)00224-4; (d) F. Caruso and M. Rossi, Mini-Rev. Med. Chem., 2004, 4, 49–60, DOI:10.2174/1389557043487565; (e) B. K. Keppler, C. Friesen, H. G. Moritz, H. Vongerichten and E. Vogel, Struct. Bonding, 1991, 78, 97 CrossRef CAS; (f) F. Caruso, M. Rossi and C. Pettinari, Expert Opin. Ther. Pat., 2001, 11, 969–979, DOI:10.1517/13543776.11.6.969; (g) C. V. Christodoulou, A. G. Eliopoulos, L. S. Young, L. Hodgkins, D. R. Ferry and D. J. Kerr, Br. J. Cancer, 1998, 77, 2088–2097, DOI:10.1038/bjc.1998.352; (h) G. Kelter, N. J. Sweeney, K. Strohfeldt, H. H. Fiebig and M. Tacke, Anti-Cancer Drugs, 2005, 16, 1091–1098, DOI:10.1097/00001813-200511000-00008; (i) P. Koepf-Maier and H. Koepf, Chem. Rev., 1987, 87, 1137–1152, DOI:10.1021/cr00081a012; (j) K. Strohfeldt and M. Tacke, Chem. Soc. Rev., 2008, 37, 1174–1187, 10.1039/b707310k.
- (a) D. Peri, S. Meker, M. Shavit and E. Y. Tshuva, Chem.–Eur. J., 2009, 15, 2403–2415, DOI:10.1002/chem.200801310; (b) D. Peri, S. Meker, C. M. Manna and E. Y. Tshuva, Inorg. Chem., 2011, 50, 1030–1038, DOI:10.1021/ic101693v; (c) T. A. Immel, M. Debiak, U. Groth, A. Buerkle and T. Huhn, ChemMedChem, 2009, 4, 738–741, DOI:10.1002/cmdc.200900038; (d) E. Y. Tshuva and D. Peri, Coord. Chem. Rev., 2009, 253, 2098–2115, DOI:10.1016/j.ccr.2008.11.015; (e) C. M. Manna, O. Braitbard, E. Weiss, J. Hochman and E. Y. Tshuva, ChemMedChem, 2012, 7, 703–708, DOI:10.1002/cmdc.201100593; (f) M. Shavit, D. Peri, C. M. Manna, J. S. Alexander and E. Y. Tshuva, J. Am. Chem. Soc., 2007, 129, 12098, DOI:10.1021/ja0753086.
- (a) S. M. Patel and L. D. Saravolatz, Med. Clin. North Am., 2006, 90, 1183, DOI:10.1016/j.mcna.2006.07.008; (b) M. L. Toews and D. B. Bylund, Proc. Am. Thorac. Soc., 2005, 2, 282–289, DOI:10.1513/pats.200504-037SR.
- V. T. Devita and P. S. Schein, N. Engl. J. Med., 1973, 288, 998–1006, DOI:10.1056/nejm197305102881905.
- (a) T. Boulikas and M. Vougiouka, Oncol. Rep., 2004, 11, 559–595 CAS; (b) R. Agarwal and S. B. Kaye, Nat. Rev. Cancer, 2003, 3, 502–516, DOI:10.1038/nrc1123.
- A. P. Jekunen, R. D. Christen, D. R. Shalinsky and S. B. Howell, Br. J. Cancer, 1994, 69, 299–306, DOI:10.1038/bjc.1994.55.
- M. Fukuda, K. Nishio, F. Kanzawa, H. Ogasawara, T. Ishida, H. Arioka, K. Bojanowski, M. Oka and N. Saijo, Cancer Res., 1996, 56, 789–793 CAS.
- (a) A. Jekunen, J. Vick, R. Sanga, T. C. K. Chan and S. B. Howell, Cancer Res., 1992, 52, 3566–3571 CAS; (b) A. M. Bergman, V. vanHaperen, G. Veerman, C. M. Kuiper and G. J. Peters, Clin. Cancer Res., 1996, 2, 521–530 CAS; (c) H. V. K. Diyabalanage, M. L. Granda and J. M. Hooker, Cancer Lett., 2013, 329, 1–8, DOI:10.1016/j.canlet.2012.09.018.
- B. T. Hennessy, R. L. Coleman and M. Markman, Lancet, 2009, 374, 1371–1382, DOI:10.1016/s0140-6736(09)61338-6.
- (a) A. G. Schultz, Chem. Rev., 1973, 73, 385–405, DOI:10.1021/cr60284a004; (b) J. F. Pizzolato and L. B. Saltz, Lancet, 2003, 361, 2235–2242, DOI:10.1016/s0140-6736(03)13780-4; (c) Y. Pommier, Nat. Rev. Cancer, 2006, 6, 789–802, DOI:10.1038/nrc1977; (d) C. J. Thomas, N. J. Rahier and S. M. Hecht, Bioorg. Med. Chem., 2004, 12, 1585–1604, DOI:10.1016/j.bmc.2003.11.036.
- (a) F. Leteurtre, J. Madalengoitia, A. Orr, T. J. Cuzi, E. Lehnert, T. Macdonald and Y. Pommier, Cancer Res., 1992, 52, 4478–4483 CAS; (b) S. D. Cline, T. L. Macdonald and N. Osheroff, Biochemistry, 1997, 36, 13095–13101, DOI:10.1021/bi971770z.
- R. J. Tallarida, F. Porreca and A. Cowan, Life Sci., 1989, 45, 947–961, DOI:10.1016/0024-3205(89)90148-3.
- N. Ganot, S. Meker, L. Reytman, A. Tzubery and E. Y. Tshuva, J. Visualized Exp., 2013, 81, e50767, DOI:10.3791/50767.
- (a) T. A. Immel, U. Groth and T. Huhn, Chem.–Eur. J., 2010, 16, 2775–2789, DOI:10.1002/chem.200902312; (b) H. Glasner and E. Y. Tshuva, J. Am. Chem. Soc., 2011, 133, 16812–16814, DOI:10.1021/ja208219f; (c) H. Glasner and E. Y. Tshuva, Inorg. Chem., 2014, 53, 3170–3176, DOI:10.1021/ic500001j; (d) S. Meker, K. Margulis-Goshen, E. Weiss, S. Magdassi and E. Y. Tshuva, Angew. Chem., Int. Ed., 2012, 51, 10515–10517, DOI:10.1002/anie.201205973; (e) S. Meker, K. Margulis-Goshen, E. Weiss, O. Braitbard, J. Hochman, S. Magdassi and E. Y. Tshuva, ChemMedChem, 2014, 9, 124–298, DOI:10.1002/cmdc.201400038.
- M. D. Hall, K. A. Telma, K.-E. Chang, T. D. Lee, J. P. Madigan, J. R. Lloyd, I. S. Goldlust, J. D. Hoeschele and M. M. Gottesman, Cancer Res., 2014, 74, 3913–3922, DOI:10.1158/0008-5472.can-14-0247.
- K. M. Buettner and A. M. Valentine, Bioinorganic Chemistry of Titanium, Chem. Rev., 2012, 112, 1863–1881, DOI:10.1021/cr1002886.
- (a) G. Mokdsi and M. M. Harding, J. Inorg. Biochem., 2001, 83, 205–209, DOI:10.1016/s0162-0134(00)00198-7; (b) U. Olszewski and G. Hamilton, Anti-Cancer Agents Med. Chem., 2010, 10, 302–311 CrossRef CAS; (c) P. Koepf-Maier, Eur. J. Clin. Pharmacol., 1994, 47, 1–16 CAS.
- (a) J. Schur, C. M. Manna, A. Deally, R. W. Koster, M. Tacke, E. Y. Tshuva and I. Ott, Chem. Commun., 2013, 49, 4785–4787, 10.1039/c3cc38604j; (b) J. Schur, C. M. Manna, E. Y. Tshuva and I. Ott, Metallodrugs, 2014, 1, 1–9, DOI:10.2478/medr-2014-0001.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13484b |
| This journal is © The Royal Society of Chemistry 2015 |