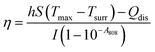Functionalized graphene/C60 nanohybrid for targeting photothermally enhanced photodynamic therapy†
Zhen Hu*ab,
Jun Lia,
Yudong Huang*a,
Lei Chena and
Zhenhui Lia
aSchool of Chemical Engineering and Technology, State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, Harbin 150001, China. E-mail: yudonghuang@163.com; huzhen@hit.edu.cn; Fax: +86-451-86402403; Tel: +86-451-86413711
bDepartment of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK
First published on 25th November 2014
Abstract
The unique structures and properties of nanocarbons will provide fascinating applications in biomedicine. Here we report a new strategy to combine C60 with graphene for targeting phototherapy. Folic acid (FA) and polyethylene glycol (PEG) were conjugated onto graphene oxide (GO) via an imide linkage. Using nucleophilic addition, the FA–GO–PEG/C60 nanohybrid was then prepared. The versatile platform of FA–GO–PEG for C60 dramatically improved tumor targeting, which has been demonstrated by a cellular uptake assay. Owing to the doping effect and the inhibition of aggregation, the hybridization process also significantly enhanced the phototoxicity of FA–GO–PEG/C60. More importantly, the photothermal therapy (PTT) of FA–GO–PEG/C60 can cause obvious cell damage, which may further enhance the photodynamic therapy (PDT) efficacy against cancer cells. As expected, the combined PDT and PTT treatment with FA–GO–PEG/C60 showed remarkably improved and synergistic effects compared to PTT or PDT alone. This study presents the potential of nanocarbons for synergistic phototherapy of cancer.
1. Introduction
Phototherapy is an emerging technique for various diseases such as cancer. The use of phototherapy as a cancer therapy is particularly attractive because of its fundamental specificity and selectivity. The treatment is a feasible method to generate localized thermal/dynamic damage to malignant cells when surgical resection is not possible.1,2 Because of their unique physicochemical properties, fullerenes (C60) and their derivatives have been investigated as candidates for phototherapy. In response to light irradiation, fullerene generates cytotoxic singlet oxygen (1O2) or other reactive oxygen species (ROS), which is the main principle of photodynamic therapy (PDT). The 1O2 production yield of C60 derivatives is even higher than those of traditional photosensitizers (PSs) such as rose bengal, methylene blue and eosin yellowish, makes it an ideal PSs used in PDT. A lot of pioneering work has focused on the use of fullerenes for PDT applications, including the cleavage of DNA strands,3,4 photoinactivation of viruses,5 production of oxidative damage to lipids,6 PDT-induced killing of tumor cells,7,8 and even a report of regressions after PDT in a mouse tumor model.9 Furthermore, a recent study suggests that multimeric C60 has potential application in photothermal therapy (PTT).10 Despite their potential, most C60 derivatives used in phototherapy have some limitations. Mainly, they are hydrophobic or easy to aggregate which leads to the decrease of singlet oxygen production and the efficiency of phototherapy. Moreover, the lack of specific target is also a principal challenge for C60 derivatives used in phototherapy.Owing to its unique physical and chemical properties,11,12 graphene may overcome the limitations of C60 used in phototherapy. A combination of these two kinds of nanocarbons will lead to remarkable synergies in certain properties because of the similarity in chemical composition and difference in geometry structure.13,14 Some studies have paid attention to the fabrication of the graphene/C60 hybrid nanomaterials. In these reports, C60 derivatives are loaded onto the graphene via a coupling reaction,15–17 nucleophilic reaction,18,19 lithiation reaction,20 π–π interaction21 and chemical vapor deposition.13,22 The results indicate that the graphene/C60 hybrid offers performance superior to that of the individual graphene and fullerene. In addition to the synergistic effect, graphene could also serve as an ideal carrier system for C60 which is high capacity loading, efficient delivery and specific targeting. Ultra-high C60 loading efficiency will be achieved owing to the extremely large surface area of graphene, which has every atom exposed on its surface.23,24 Once the C60 is coated on the graphene surface, its free movement is restricted. This effect will prevent the spontaneous aggregation of the C60 and its derivatives, and make them stable in the solution. On the other hand, the presence of C60 on a graphene sheet surface can effectively prevent the aggregation of the latter which in turn helps the dispersion of the former.15,19 For targeting gene and drug delivery, much work has been carried out using graphene as the efficient nanocarrier.25–27 A wise strategy to let graphene exhibits tumor targeting is to conjugate with specific ligands that can recognize the cancer cell or magnetic nanoparticles.28,29 Therefore, graphene has shown great promise as a novel targeting delivery system for C60 used in phototherapy.
Apart from the excellent drug carrier, graphene also can act as a photothermal agent and convert near infra red (NIR) light into heat efficiently and thus induce hyperthermia to cells and surrounding tissues.30–36 In recent years, graphene is also used for combination cancer therapy. According to immobilize anticarcinogen or PDT PSs, several graphene based nanomaterials have shown excellent anti-cancer performance for combined chemotherapy/PTT37–39 and PDT/PTT.40–43 Previous studies have indicated that, combining C60 and graphene in one system can exceed the individual phototherapeutic response of each nanocarbon and may lead to enhanced therapeutic outcome. However, it is still a great challenge to develop facile and efficient synthetic approaches for graphene/C60 nanohybrid, which is specific targeting, biocompatibility and could effectively show hybridization effects between graphene and C60. Moreover, to the best of our knowledge, while photodynamic activities of C60 and photothermal anticancer effects of graphene have been well-documented, the possibility of direct tumor cell killing by graphene/C60 hybrids has remained largely unexplored. Herein, we design and develop a multifunctional graphene/C60 nanohybrid, which is synthesized by covalently grafting folic acid (FA) and polyethylene glycol (PEG) with graphene oxide (GO) via coupling reaction, and then pristine C60 is loaded on the surface of graphene via the nucleophilic addition. Benefiting from the unique nanostructure, the hybrid integrates the multifunctions of PDT and PTT into a single nanoplatform, which is expected to improve the therapeutic efficiency.
2. Methods and materials
2.1. Materials
Chloroacetic acid, NaOH and FA were product of Sinoreagent Co. Ltd. C60 was purchased from Wuhan University. Six-Armed PEG-amine (10 kDa) was provided by SunBio Inc. The N-hydroxysuccinimide (NHS), 2-(N-morpholino)ethanesulfonic acid (MES), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) were provided by J&K Scientific Ltd. RMPI 1640 medium and the fetal calf serum were provided from Gibco BRL. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl (MTT) was provided from Sigma. 2,7-Dichlorofluorescein diacetate (DCF-DA) was obtained from Molecular Probe Inc. Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit was obtained from Biosea Biotechnology Co. Ltd.2.2. Synthesis of FA–GO–PEG
In our previous work, we have reported the synthesis of FA–GO.28 In the present work, FA–GO was further modified by 6-armed PEG-amine for loading C60, resulting in the synthesis of FA–GO–PEG. In brief, GO was carboxylated by ClCH2COOH to obtain GO–COOH. Then GO–FA was covalently conjugated with the carboxylic acid group of GO–COOH using EDC/NHS chemistry. The resulting GO–FA was suspended in 20 mL water to give a concentration of 1 mg mL−1. Then 100 mg EDC was added to the suspension and the mixture was sonicated for 30 min. 6-Armed PEG-amine (100 mg) was added in the mixture, allowed to react overnight at room temperature. The product (FA–GO–PEG) was purified by centrifugation (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm, 10 min) and washed several times with deionized water to remove unreacted PEG and other reagents.
800 rpm, 10 min) and washed several times with deionized water to remove unreacted PEG and other reagents.
2.3. Preparation of FA–GO–PEG/C60 nanohybrid
FA–GO–PEG (20 mg) was dispersed in 20 mL DMSO with the aid of sonication for 30 min. Then 50 mg C60 in 20 mL toluene was added into above solution. The amino groups on FA–GO–PEG could undergo nucleophilic addition across the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in C60.44,45 The mixture was stirred at 90 °C for 2 days and was filtered and washed with toluene and ethanol sequentially for several times to completely removing excess C60. Followed by drying at 80 °C under vacuum for 12 h, 24 mg FA–GO–PEG/C60 nanohybrid product was obtained.
C bonds in C60.44,45 The mixture was stirred at 90 °C for 2 days and was filtered and washed with toluene and ethanol sequentially for several times to completely removing excess C60. Followed by drying at 80 °C under vacuum for 12 h, 24 mg FA–GO–PEG/C60 nanohybrid product was obtained.
2.4. Characterization
Fourier transform infrared spectra (FT-IR, Bruker, Tensor 27), Raman spectra (Horiba Jobin Yvon, France), and UV-visible light absorption spectra (UV-vis, Beijing Purkinje General Instrument Co., Ltd., TU-1901) were used to characterize the functionalized graphene. Thermogravimetric analysis (TG) was run under a nitrogen flow (40 mL min−1) using a Q500 TG instrument (TA Instruments). The samples were heated from room temperature to 400 °C at a ramp rate of 5 °C min−1. Transmission electron microscopy (TEM, JEOL, JEM-2100F) and atomic force microscope (AFM, Sounding Housing SPA 400) were used to characterize the morphology and structure of the samples. Two optical-fiber coupled power-tunable diode lasers (continuous wave) with wavelengths at 532 nm (maximal power = 2 W) and 808 nm (maximal power = 7 W) were both purchased from Beijing Stone Laser Ltd. The exact optical powers of lasers used in the experiments were calibrated and measured by a LPE-1B laser power/energy meter (Physcience Opto-Electronics Co. Ltd. Beijing).2.5. Singlet oxygen generation in aqueous solutions
To measure the singlet oxygen (1O2) generation by FA–GO–PEG/C60, p-nitroso-N,N′-dimethylaniline (RNO) was used as an indicator. Solutions of GO, folic acid C60 derivative (FFA) or FA–GO–PEG/C60 were mixed with RNO (25 μM), imidazole (25 μM), and phosphate-buffered saline (PBS, 10 mM, pH = 7.4) and then irradiated by 532 nm laser at the light power density of 0.1 W cm−2 for different periods of time. The FFA served as comparison was synthesized by the method mentioned in our previous work.46 The generation of 1O2 would result in the reduction of RNO absorption at 440 nm, which reflects the production of 1O2.2.6. Photothermal activity of FA–GO–PEG/C60 nanohybrid
Solutions of GO, FFA or FA–GO–PEG/C60 (10 μg mL−1) were taken in a 1 mL quartz sample cell. The solution was then illuminated with 808 nm NIR laser for different time periods with a power density of 2 W cm−2. The increase in temperature was measured by a thermocouple immersed into suspension.2.7. Cell treatment and cytotoxicity assay
HeLa cells were cultured in an atmosphere with 5% CO2 and at 37 °C provided by a NAPCO CO2 incubator in RMPI 1640 medium containing 10% heat-inactivated fetal calf serum. Then, the culture medium was replaced by the fresh medium containing 2% fetal calf serum and specific nano-materials. After 24 h of incubation, the cells were washed twice with PBS and irradiated using 808 nm NIR laser (2 W cm−2, 3 min) for PTT treatment. The method of detecting the effect of PDT treatment was the same as that used in PTT treatment, except that 532 nm laser was used for irradiation (0.1 mW cm−2, 5 min). For synergistically enhanced anti-cancer effect of the combination of PDT and PTT, the cells were irradiated using the 808 nm laser at a power density of 2 W cm−2 for 3 min (360 J cm−2). After photothermally heating, cells were immediately irradiated by the 532 nm laser at a power density of 0.1 W cm−2 for 5 min (30 J cm−2).After another 24 h incubation, the cell viabilities of samples were determined by the MTT assay. In brief, HeLa cells (1 × 105 per mL) were seeded in 96-well plates. 10 μL MTT solutions (final concentration, 0.5 mg mL−1) were added and incubated for additional 4 h. The lysis buffer (20% sodium dodecylsulfate in 50% aqueous N,N-dimethylformamide) was added to solubilize the formazan crystal, and absorbance at 570/630 nm was measured with a microplate reader (Molecular Devices).
2.8. In vitro cellular uptake assay
HeLa cells were cultured in 24 well tissue plate at a density of 1 × 105 cells per well. FITC was loaded on FA–GO–PEG/C60 by sonicating FITC solution (0.05 mg mL−1, 2 mL) with an aqueous suspension of FA–GO–PEG/C60 (2.0 mg mL−1, 1 mL) for 30 min to mix them together, followed by stirring in the dark for 12 h. Unbound FITC was removed by rinsing and centrifugation. As a comparison, GO and FA–GO were treated with FITC by exactly the same steps. Then, HeLa cells were exposed to GO–FITC, FA–GO–FITC and FA–GO–PEG/C60–FITC with the final concentration of 10 μg mL−1 at 37 °C for certain time, respectively. The cells were harvested and resuspended in PBS after washed for 3 times. To investigate the targeted uptake of FA–GO–PEG/C60 by HeLa cells, cells were observed by confocal fluorescence microscopy equipped with 450–490 nm band-pass filter.2.9. Measurement of intracellular ROS accumulation
The fluorescent probe DCF-DA was used to monitor the intracellular accumulation of ROS. After treatment, cells (1 × 106 cells per 3 mL in 6-well plates) were rinsed with D-Hanks solution and 10 μM DCF-DA was loaded. After 20 min incubation at 37 °C, the cells were harvested after being washed with PBS 3 times. The intracellular ROS accumulation was measured by using a Becton–Dickinson fluorescence-activated cell analyzer while data analysis was performed with Modifit LT 2.0 (Becton–Dickinson). About 1 × 104 cells were counted for each analysis. The fluorescence intensity was quantified with CELLQuest software.2.10. Determination of apoptosis
Apoptotic cell death was analyzed by double staining with annexin V-FITC and propidium iodide (PI). Apoptosis of cells was evaluated using an annexin-V FITC apoptosis detection kit. Cells were harvested, washed and incubated at 4 °C for 30 min in the dark with annexin V-FITC and PI, then were analyzed on a FACS Vantage SE flow cytometer (Becton–Dickinson).2.11. Statistical analysis
Values were reported as means ± S.D. Statistical comparisons were made by one-way ANOVA to detect significant difference using SPSS 13.0 for windows.3. Results and discussion
3.1. Synthesis and characterization of FA–GO–PEG/C60 nanohybrid
In this work, we design and employ a facile strategy to prepare FA–GO–PEG/C60 nanohybrid via a three-step reaction using GO as the starting material. The notable difference from the previous work is that the nanocarbon hybrid would theoretically be expected to improve the targeting, biocompatibility and the phototherapy effects. The synthetic route to FA–GO–PEG/C60 is explained in Scheme 1.As shown in Fig. 1a, noticeable absorptions are observed at ∼3406 cm−1 (O–H), 2898 cm−1 (C–H), 1715 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1630 cm−1 (C
O), 1630 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching) in the IR spectrum of GO. In the C60 spectra, it shows several characteristic absorption peaks, respectively located at 1436, 1185, 577 and 527 cm−1. The IR spectra of GO and FA–GO are almost same, however, the characteristic peak of FA at 1648 cm−1 are clearly observed in the GO–FA spectra. This suggests that FA is successfully conjugated onto the GO surface. For FA–GO–PEG/C60, the characteristic peaks of GO at ∼3406, 2989 and 1715 cm−1 are observed. Moreover, the characteristic absorption peaks of C60 (1436, 1185, 527 cm−1) and FA (1648 cm−1) are simultaneously exist in its IR spectrum, confirming that the FA–GO–PEG/C60 have been successfully prepared.
C stretching) in the IR spectrum of GO. In the C60 spectra, it shows several characteristic absorption peaks, respectively located at 1436, 1185, 577 and 527 cm−1. The IR spectra of GO and FA–GO are almost same, however, the characteristic peak of FA at 1648 cm−1 are clearly observed in the GO–FA spectra. This suggests that FA is successfully conjugated onto the GO surface. For FA–GO–PEG/C60, the characteristic peaks of GO at ∼3406, 2989 and 1715 cm−1 are observed. Moreover, the characteristic absorption peaks of C60 (1436, 1185, 527 cm−1) and FA (1648 cm−1) are simultaneously exist in its IR spectrum, confirming that the FA–GO–PEG/C60 have been successfully prepared.
Fig. 1b gives UV-vis absorption spectra of GO, FA–GO and FA–GO–PEG/C60 in H2O. GO shows an absorption band at 228 nm, and the conjugation of FA to GO is confirmed by UV-vis spectra. A peak at 228 nm disappeared while a new peak at ∼281 nm appeared due to the presence of FA in the FA–GO. C60 shows three typical absorption peaks located at 215, 265, 335 nm in n-hexane (Fig. S1†). As water is more polar than n-hexane, the absorption peaks of C60 will show red shifts in water environment. The peak at ∼280 nm of FA–GO–PEG/C60 may both due to the characteristic peak of C60 (ref. 17) and FA. Meanwhile, FA–GO–PEG/C60 shows strong absorbance at 200–400 nm, which may lead overlapping the characteristic peaks of C60. The FA–GO–PEG/C60 solution showed increased optical absorption values in the NIR and visible wavelengths (Fig. 2b). This increased optical absorption may attribute to the hydrolysis of esters and opening of epoxide groups on the GO sheets under basic conditions during the carboxylation process.41 Meanwhile, since all the samples have the same concentration and light path-length, the increase may also due to doping effect13 and the higher molar absorption coefficient of FA–GO–PEG/C60. Owing to this enhancement of absorption, FA–GO–PEG/C60 could be an excellent PS, thereby achieving improved synergistic PTT/PDT effects.
 | ||
| Fig. 2 (a) TEM image of GO, (b) TEM image of FA–GO–PEG/C60, (c) AFM image of GO with height profile diagram, (d) AFM image of FA–GO–PEG/C60 with height profile diagram. | ||
Raman spectra of the GO, C60, FA–GO and FA–GO–PEG/C60 are also conducted, as shown in Fig. 1c. As expected, the GO shows an intense tangential mode (G band) at 1581 cm−1, with a disordered-induced peak (D band) at 1368 cm−1, while C60 shows the Ag-breathing and the Ag-pinch modes at 478 cm−1 and 1458 cm−1, and additional Hg mode at 1523 cm−1. Since the intensities for some Hg-modes are very weak in the room temperature spectra, some Hg lines of C60 aren't detected in the present study. For the FA–GO and FA–GO–PEG/C60, peaks are clearly observed at 1368 and 1581 cm−1, which can be assigned to the D and G bands of the graphene. The peak at 2724 cm−1 corresponding to the overtone of the D band and the peak at 2951 cm−1 associating with the D + G band also indicates that increased disorder has appeared in graphene. The D-band to G-band intensity ratio (ID/IG) has been widely used as a measure for the disorder or the extent of covalent modification of the graphene surface. In the present work, a slight increase in the ID/IG ratio from 0.68 for the GO to 0.77 for FA–GO is observed. Moreover, the ID/IG of the FA–GO (0.77) is lower than FA–GO–PEG/C60 (0.83). The results indicate that the grafting of FA, PEG and C60 onto graphene produces more defects and introduces more functional groups. The small peak at 491 cm−1 in the FA–GO–PEG/C60 Raman spectrum should be assigned to the Ag-breathing mode of the C60, which indicate the successful grafting of C60. This Ag peak is shifted by 13 cm−1 compared with the pure C60, which suggests a strong interaction between the C60 cage and the GO sheet.16 To further demonstrate the attachment of C60, the fluorescence spectra of FA–GO–PEG/C60 are provided (Fig. S2†). The results show week diagnostic fluorescence (excitation wavelength: 352 nm; emission wavelength: 438 nm) generated by C60 component, which confirm the loading of C60 to the graphene sheets.
To quantitatively determine the attaching of C60, TG analysis is employed. The initial degradation temperature of GO (the temperature at 10 wt% mass loss, Ti) is 210 °C, and the mass residue is 59.0 wt% at 600 °C (Fig. 1d). After modified by FA and PEG, the Ti of FA–GO–PEG is delayed to 238 °C and its residue increases to 66.3 wt%. As the pristine C60 is thermally stable, its mass residue is 98.1 wt% at 600 °C. Due to the higher thermal stability of C60, the Ti of FA–GO–PEG/C60 is 300 °C, while the mass residue at 600 °C is significantly increases to 74.5 wt%. By comparing the mass residue of C60, FA–GO–PEG, and FA–GO–PEG/C60, the content of C60 in FA–GO–PEG/C60 can be calculated, which is 25.5 wt%.
The morphology of GO and FA–GO–PEG/C60 is characterized by TEM. As can be seen (Fig. 2a), the GO displays flake-like shapes with wrinkles and frees from any particulate contamination. By contrast, the corresponding TEM image for the FA–GO–PEG/C60 given in Fig. 2b clearly shows the surface grafted by particles with an average size of 2–5 nm characteristic of C60 aggregates. To observe and characterize the topography of the GO and FA–GO–PEG/C60, AFM is applied as a suitable technique, as shown in Fig. 2c and d. Analysis of GO by AFM reveals the GO with ∼0.81 nm in topographic height (Fig. 2c), which is in good consistency with the typical thickness of the observed single-layer GO (∼0.8 nm). The sheet thickness of FA–GO–PEG/C60 (∼3.29 nm) is much higher than that of GO (Fig. 2d), likely owing to the covalent PEGylation that offers more condensed surface polymer coating on GO surface. In addition, the size of the C60 particles is measured ∼2 nm. The TEM and AFM results confirm the attachment of the C60 on the surface of GO platelets.
As a matter of fact, C60 is not able to solve in H2O but solve in some nonpolar or low-polar solvents such as toluene. Modification of C60 with hydrophilic addend produces an amphiphilic molecule, which tends to form aggregates, such as vesicles, rods, globules, membranes, and linear assemblies. Our previous study has demonstrated that the aggregate size influences the biological activities of water-soluble C60 derivatives.44–46 Generally speaking, the aggregation of C60 would reduce surface-to-volume, enhance steric hindrance, and reduce the intracellular content, which further reduces the biological activities of C60. In the present study, GO sheet is decorated with C60 with a size of ∼2 nm. It seems that the nucleophilic addition firmly fixes the C60 on the graphene, which makes C60 unable to move freely and can't form large aggregates. In this way, C60 will exert its biological activities more effectively.
3.2. Photodynamic and photothermal properties of FA–GO–PEG/C60 nanohybrid
Singlet oxygen generation is the critical step in PDT. In this work, the 1O2 generation by GO, FFA and FA–GO–PEG/C60 is measured under irradiation by a 532 nm laser (Fig. 3a). As expected, GO does not show any 1O2 production as there is no change in the absorption with increasing laser exposure time. FA–GO–PEG/C60 shows a sharp decrease in RNO absorption with increasing time of laser exposure, indicating rapid generation of 1O2. Our previous work has been demonstrated that the FFA could generate 1O when photoexcited.46 In the present study, the FA–GO–PEG/C60 shows similarly rapid absorption bleaching compared to the FFA, which indicates that the 1O2 production ability by FA–GO–PEG/C60 is substantially high (80–90% of FFA). It has been shown previously that the GO would act as efficient quenchers, which significantly reduce the 1O2 production of PSs loaded on the GO.41,42 In the present work, however, the 1O2 production ability of FA–GO–PEG/C60 is similar with FFA. It seems that the quenching effect of 1O2 production from C60 by FA–GO–PEG appears to be less drastic compared to previous study, and the reason may relate to the unique structure and properties of C60 and graphene. On one hand, the aggregation of FFA (∼120 nm) reduces the 1O2 generation ability of C60 core.46 On the other hand, owing to the doping effect and aggregation prevention effect of GO, the C60 on the FA–GO–PEG sheets will be more effective in light absorbing and produce more 1O2. The excellent 1O2 generation ability of C60 attached on graphene allows us to use FA–GO–PEG/C60 for PDT treatment.The photothermal properties of FA–GO–PEG/C60 are evaluated in the present study. Since PTT does not require oxygen to interact with the target cells or tissues, it is able to use longer wavelength light, which is less energetic and therefore less harmful. The biological tissues also exhibit a deep penetrability with very low absorption of NIR photons in the wavelength range of 700 to 1100 nm. Thus, 808 nm laser is selected as excitation light source in the present study. While the temperature of water only increases 2.3 °C after 808 nm laser irradiation, the solution temperature of FA–GO–PEG/C60 rises rapidly from 26 °C to 44.8 °C in 3 min, showing a significant temperature increase of 18.8 °C (Fig. 3b). It is worth noting that, the water soluble C60 derivative-FFA converts the NIR light radiation to vibrational energy to elevate the temperature for 6.4 °C. The photothermal activity of C60 shows great promise to achieve excellent synergistic phototherapy effects of FA–GO–PEG/C60. Following Roper's report,47 the photothermal conversion efficiency of FA–GO–PEG/C60, η, is calculated using the following equation.
3.3. FA–GO–PEG/C60 induced phototoxicity and apoptosis in HeLa cells
Phototoxicity of PDT, PTT and PDT/PTT combined treatment on HeLa cells are evaluated in the present study. Without light irradiation, GO, FFA and FA–GO–PEG/C60 does not show any significant dark toxicity towards HeLa cells (Fig. 4a). The cell viability is still 98.4% after treated with 20 μg mL−1 FA–GO–PEG/C60 in dark (Fig. 4b), which proves that FA–GO–PEG/C60 is cytocompatible. As for the effect of PTT, the 808 nm laser irradiation with 10 μg mL−1 GO and FA–GO–PEG/C60 reduces the cell viability to 80.4% and 74.6% respectively, demonstrating the photothermal activities of graphene sheets (Fig. 4a). As for the effect of PDT, the cell viability of group FFA and FA–GO–PEG/C60 decreases to 72.4% and 35.4% after irradiation of 532 nm laser, respectively (Fig. 4a). It seems that the cells are more sensitive to PDT in the present experimental conditions. The 808 nm laser irradiation at a low power only generates mild heating, which induces small amount of cell death. However, the light-induced local heating of graphene sheets may cause damage to the cell membrane, which further increases cellular uptake and makes 1O2 attacks the cells more easily. As expected, when the PTT and PDT are combined, the cell viability was remarkably reduced to 3.1% after treated with 10 μg mL−1 FA–GO–PEG/C60, which is evidently much lower than that by the individual ones (Fig. 4a). In the present study, we also cultures HeLa cells with FA–GO–PEG/C60 at different concentrations for MTT assay (Fig. 4b). Our results indicate that with the increasing concentrations of FA–GO–PEG/C60, the lethality increases, suggesting a dose-dependent effect in vitro. The cytotoxicity analysis shows that 0–20 μg mL−1 GO, FA–GO and FA–GO–PEG/C60 doesn't cause obvious cell death in dark, which indicates the biocompatibility of the nanocarbons (Fig. S4†). Besides, our observations demonstrate that the laser light irradiation doesn't cause significant cytotoxicity, which confirms the phototoxicity of FA–GO–PEG/C60 are result from its ability to generate 1O2 and heating when photoexcited.It is interesting to notice that the PDT effect of FA–GO–PEG/C60 is much better than FFA, although their light-induced 1O2 generation performances are similar (Fig. 4a). To understand why this happens, cellular uptake and intracellular ROS accumulation are analyzed.
Fig. 5 shows the fluorescence images of HeLa cells after being incubated with GO–FITC, FA–GO–FITC and FA–GO–PEG/C60–FITC, respectively. Much stronger fluorescence can be seen in the HeLa cells after incubation with FA–GO–FITC than GO–FITC, which suggests specific targeting of FA–GO under the leading of FA molecules. In the present study, FA–GO–PEG/C60–FITC shows stronger fluorescence inside cells than FA–GO–FITC. Graphene sheets functionalized with PEG exhibit high solubility and stability in physiological solutions, which would enhance the endocytosis of FA–GO–PEG/C60 by cells. The corresponding intracellular fluorescence intensity is also given in Fig. 6a to make a quantitative comparison. The fluorescence intensity of FA–GO–PEG/C60 group (1082) is much higher than GO (303) and FA–GO (746) after 24 h incubation. The result indicates that the FA–GO–PEG/C60 can be quickly and effectively delivered into the targeted tumor cells. It is assumed that the enhanced uptake would make the FA–GO–PEG/C60 kills tumor cells more effectively, and the intracellular ROS measurement proves the speculation. Accumulation of intracellular ROS is detected by using DCF-DA (Fig. 6b). The intracellular ROS generation presents the same trend as the cell viability decrease. The more intracellular ROS generated, the more decrease in cell viability. The results indicate that anti-cancer activity of FA–GO–PEG/C60 is associated with the intracellular ROS production. The higher uptake of FA–GO–PEG/C60 based on the folate receptor and PEG-induced endocytosis transports more C60 molecule into the cells, which exerts the phototoxicity inside the cells and produces the enhanced phototherapy effects. The Fig. 6b also indicates that the intracellular ROS accumulation irradiated by 808 + 532 nm laser is higher than that of only 532 nm. The PTT effects cause overheating to cells, which induce cell death and apoptosis. ROS play an important role in the process of apoptosis in many cell types. Increased ROS generation is a key phenomena, which can be frequently observed in cells subjected to anticancer drugs treatment, that is, accumulation of ROS inside the cell often signalizes apoptosis.49 The previous study has also indicated that PTT by NIR-excited graphene nanoparticles triggers apoptotic/necrotic cancer cell death associated with ROS.50 While irradiated by 808 + 532 nm laser, the intracellular ROS level is both related with PDT and PTT effects. The cell damage caused by PTT elevates the ROS level in the cell, which further enhances the PDT effects of FA–GO–PEG/C60.
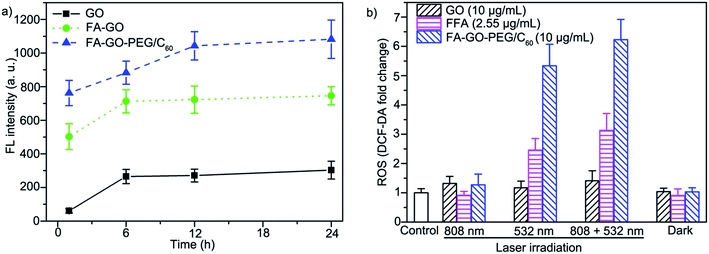 | ||
| Fig. 6 (a) The corresponding fluorescence intensities of HeLa cells in Fig. 5; (b) intracellular ROS generation by GO, FFA and FA–GO–PEG/C60. HeLa cells are exposed to 808 nm laser (light dose: 360 J cm−2), 532 nm laser (light dose: 30 J cm−2), or 808 nm and 532 nm laser for light irradiation, respectively. Cells incubated with GO, FFA and FA–GO–PEG/C60 without irradiation served as dark group. The fluorescent probe DCF-DA was used to monitor the intracellular accumulation of ROS. The results from representative of at least three experiments are presented. Values are presented as mean ± S.D. (n = 3). | ||
To explore the possible cell death mechanism, double staining of annexin V and PI is performed in the presence of laser irradiation. Cells are stained with annexin V which reacts against externalized phosphatidylserine, a characteristic of apoptotic cells, and PI dye which stains both late-stage apoptotic and necrotic cells displaying permeable membranes. Fig. 7 shows the dot plots of the flow cytometry analysis. In control group, cells show only very weak staining with annexin V and PI (Fig. 7a). As expected, the change trend of FA–GO–PEG/C60 induced apoptosis is the same as cell viability. The PTT and PDT combining therapies remarkably increase the percentage of apoptosis to 54.27%, which is evidently better than the individual therapy (Fig. 4a). Meanwhile, the results indicate that the cell apoptosis or death is mainly dependent on the PDT effect, and apoptotic cell death is predominant in all experiments. The apoptosis, a preferred mode of killing the cancer cells in cancer therapy, is induced synergistically, resulting in distinguished improvement in phototherapy activities.
Based on the above study, the hypothetical mechanism of PTT and PDT combined therapy of FA–GO–PEG/C60 is shown in Scheme 2. The combination of these two inconceivable nanocarbons leads to remarkable synergies in phototherapy. First, the hybridization process prevents the restacking of graphene and the aggregation of C60, which supply more surface active sites and increase the PDT efficiency of FA–GO–PEG/C60. Second, owing to the doping effect, the FA–GO–PEG/C60 exhibits boarder absorption range and higher absorbance. Third, the PTT effects of FA–GO–PEG/C60 cause obvious cell damage and achieve synergistic enhancement of phototherapy effects. Fourth, the graphene carrier equipped with folic acid and PEG has specific targeting to tumor cells, which significantly enhances the cellar uptake of FA–GO–PEG/C60. Once the FA–GO–PEG/C60 is taken by tumor cells, it causes heating and significant intracellular ROS production under light irradiation. The photo generated ROS and heating cause a marked decrease in cell survival and elevation of oxidative stress. Finally, the phototherapy effects of FA–GO–PEG/C60 apparently involves induction of the “programmed” cell death, known as apoptosis. The work provides a facile way to synthesize novel graphene/C60 nanohybrid with excellent therapeutic efficiency for cancer. It should be valuable for developing highly effective nanocarbons-based cancer theranostics.
 | ||
| Scheme 2 The hypothetical mechanism of synergistic enhancement of FA–GO–PEG/C60 in combined PTT and PDT. | ||
4. Conclusions
In conclusion, we have developed a facile and efficient synthetic route to prepare a novel FA–GO–PEG/C60 nanohybrid that combines the multiple functions and advantages of C60 and graphene. In the present work, FA and PEG functionalized graphene is used as tumor targeting nanocarrier for C60, which offers dramatically improved cell uptake. Furthermore, the hybridization process enhances the light absorption properties, prevents the restacking of graphene, and limits the aggregation of C60, thereby shows an enhanced phototherapy effect of FA–GO–PEG/C60. After light irradiation, FA–GO–PEG/C60 causes a marked decrease in cell survival and elevation of oxidative stress, and induces apoptotic death. Compared with PDT or PTT alone, the combined treatment with FA–GO–PEG/C60 shows higher cell apoptosis and death, indicating a synergistic effect of combination phototherapy. Our work reveals that the hybridization of the two nanocarbons represents a promising biomedicine for cancer therapy.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 51103031), the Research Fund for the Doctoral Program of Higher Education of China (no. 20112302120034), the Special Foundation of China Postdoctoral Science (no. 201003436), the Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (no. QA201415), the Fundamental Research Funds for the Central Universities (no. HIT. NSRIF. 2012034), and the China Scholarship Council (no. 201303070100).Notes and references
- Z. Liu, J. T. Robinson, S. M. Tabakman, K. Yang and H. Dai, Mater. Today, 2011, 14, 316–323 CrossRef CAS.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS PubMed.
- N. Singh, B. Manshian, G. J. Jenkins, S. M. Griffiths, P. M. Williams, T. G. Maffeis, C. J. Wright and S. H. Doak, Biomaterials, 2009, 30, 3891–3914 CrossRef CAS PubMed.
- Y. Liu, Y.-L. Zhao, Y. Chen, P. Liang and L. Li, Tetrahedron Lett., 2005, 46, 2507–2511 CrossRef CAS PubMed.
- V. V. Zarubaev, I. M. Belousova, O. I. Kiselev, L. B. Piotrovsky, P. M. Anfimov, T. C. Krisko, T. D. Muraviova, V. V. Rylkov, A. M. Starodubzev and A. C. Sirotkin, Photodiagn. Photodyn. Ther., 2007, 4, 31–35 CrossRef PubMed.
- J. P. Kamat, T. P. A. Devasagayam, K. I. Priyadarsini and H. Mohan, Toxicology, 2000, 55–61 CrossRef CAS.
- F. Rancan, S. Rosan, F. Boehm, A. Cantrell, M. Brellreich, H. Schoenberger, A. Hirsch and F. Moussa, J. Photochem. Photobiol., B, 2002, 67, 157–162 CrossRef CAS.
- P. Mroz, A. Pawlak, M. Satti, H. Lee, T. Wharton, H. Gali, T. Sarna and M. R. Hamblin, Free Radical Biol. Med., 2007, 43, 711–719 CrossRef CAS PubMed.
- F. Jiao, Y. Liu, Y. Qu, W. Li, G. Zhou, C. Ge, Y. Li, B. Sun and C. Chen, Carbon, 2010, 48, 2231–2243 CrossRef CAS PubMed.
- D. J. Lee, Y. S. Ahn, Y. S. Youn and E. S. Lee, Polym. Adv. Technol., 2013, 24, 220–227 CrossRef CAS.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- E. K. Jeon, C. S. Yang, Y. Shen, T. Nakanishi, D. S. Jeong, J. J. Kim, K. S. Ahn, K. J. Kong and J. O. Lee, Nanotechnology, 2012, 23, 455202–455207 CrossRef PubMed.
- Z. B. Liu, Y. F. Xu, X. Y. Zhang, X. L. Zhang, Y. S. Chen and J. G. Tian, J. Phys. Chem. B, 2009, 9681–9686 CrossRef CAS PubMed.
- Y. Zhang, L. Ren, S. Wang, A. Marathe, J. Chaudhuri and G. Li, J. Mater. Chem., 2011, 21, 5386–5391 RSC.
- X. Zhang, Y. Huang, Y. Wang, Y. Ma, Z. Liu and Y. Chen, Carbon, 2009, 47, 334–337 CrossRef CAS PubMed.
- Z. B. Liu, Y. F. Xu, X. Y. Zhang, X. L. Zhang, Y. S. Chen and J. G. Tian, J. Phys. Chem. B, 2009, 113, 9681–9686 CrossRef CAS PubMed.
- J. Pu, Y. Mo, S. Wan and L. Wang, Chem. Commun., 2014, 50, 469–471 RSC.
- P. Song, L. Liu, G. Huang, Y. Yu and Q. Guo, Nanotechnology, 2013, 24, 505706–505713 CrossRef PubMed.
- D. Yu, K. Park, M. Durstock and L. Dai, J. Phys. Chem. Lett., 2011, 2, 1113–1118 CrossRef CAS.
- T. Gan, C. Hu, Z. Sun and S. Hu, Electrochim. Acta, 2013, 111, 738–745 CrossRef CAS PubMed.
- M. Chen, H. Zhou, F. Yu, H. Yang, G. Wang, J. He and L. Sun, Nanoscale, 2013, 5, 8359–8362 RSC.
- Z. Liu, J. T. Robinson, X. Sun and H. Dai, J. Am. Chem. Soc., 2008, 10876–10877 CrossRef CAS PubMed.
- X. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203–212 CrossRef CAS PubMed.
- A. J. Shen, D. L. Li, X. J. Cai, C. Y. Dong, H. Q. Dong, H. Y. Wen, G. H. Dai, P. J. Wang and Y. Y. Li, J. Biomed. Mater. Res., Part A, 2012, 100, 2499–2506 Search PubMed.
- Y. Yang, Y. M. Zhang, Y. Chen, D. Zhao, J. T. Chen and Y. Liu, Chem.–Eur. J., 2012, 18, 4208–4215 CrossRef CAS PubMed.
- L. Zhang, J. Xia, Q. Zhao, L. Liu and Z. Zhang, Small, 2010, 6, 537–544 CrossRef CAS PubMed.
- Z. Hu, J. Li, C. Li, S. Zhao, N. Li, Y. Wang, F. Wei, L. Chen and Y. Huang, J. Mater. Chem. B, 2013, 1, 5003–5013 RSC.
- X. Yang, X. Zhang, Y. Ma, Y. Huang, Y. Wang and Y. Chen, J. Mater. Chem., 2009, 19, 2710–2714 RSC.
- K. Yang, S. Zhang, G. Zhang, X. Sun, S. T. Lee and Z. Liu, Nano Lett., 2010, 10, 3318–3323 CrossRef CAS PubMed.
- O. Akhavan, E. Ghaderi and H. Emamy, J. Mater. Chem., 2012, 22, 20626–20633 RSC.
- S. H. Hu, Y. W. Chen, W. T. Hung, I. W. Chen and S. Y. Chen, Adv. Mater., 2012, 24, 1748–1754 CrossRef CAS PubMed.
- X. Ma, H. Tao, K. Yang, L. Feng, L. Cheng, X. Shi, Y. Li, L. Guo and Z. Liu, Nano Res., 2012, 5, 199–212 CrossRef CAS.
- K. Yang, L. Hu, X. Ma, S. Ye, L. Cheng, X. Shi, C. Li, Y. Li and Z. Liu, Adv. Mater., 2012, 24, 1868–1872 CrossRef CAS PubMed.
- D. K. Lim, A. Barhoumi, R. G. Wylie, G. Reznor, R. S. Langer and D. S. Kohane, Nano Lett., 2013, 13, 4075–4079 CrossRef CAS PubMed.
- J. Chen, X. Wang and T. Chen, Nanoscale Res. Lett., 2014, 9, 86–95 CrossRef PubMed.
- H. Kim, D. Lee, J. Kim, T. Kim and W. J. Kim, ACS Nano, 2013, 7, 6735–6746 CrossRef CAS PubMed.
- X. C. Qin, Z. Y. Guo, Z. M. Liu, W. Zhang, M. M. Wan and B. W. Yang, J. Photochem. Photobiol., B, 2013, 120, 156–162 CrossRef CAS PubMed.
- H. W. Yang, Y. J. Lu, K. J. Lin, S. C. Hsu, C. Y. Huang, S. H. She, H. L. Liu, C. W. Lin, M. C. Xiao, S. P. Wey, P. Y. Chen, T. C. Yen, K. C. Wei and C. C. Ma, Biomaterials, 2013, 34, 7204–7214 CrossRef CAS PubMed.
- B. Tian, C. Wang, S. Zhang, L. Feng and Z. Liu, ACS Nano, 2011, 5, 7000–7009 CrossRef CAS PubMed.
- A. Sahu, W. I. Choi, J. H. Lee and G. Tae, Biomaterials, 2013, 34, 6239–6248 CrossRef CAS PubMed.
- Y. Wang, H. Wang, D. Liu, S. Song, X. Wang and H. Zhang, Biomaterials, 2013, 34, 7715–7724 CrossRef CAS PubMed.
- G. Gollavelli and Y. C. Ling, Biomaterials, 2014, 35, 4499–4507 CrossRef CAS PubMed.
- Z. Hu, W. Guan, W. Wang, L. Huang, X. Tang, H. Xu, Z. Zhu, X. Xie and H. Xing, Carbon, 2008, 46, 99–109 CrossRef CAS PubMed.
- Z. Hu, Y. Huang, W. Guan, J. Zhang, F. Wang and L. Zhao, Biomaterials, 2010, 31, 8872–8881 CrossRef CAS PubMed.
- Z. Hu, C. Zhang, Y. Huang, S. Sun, W. Guan and Y. Yao, Chem.-Biol. Interact., 2012, 195, 86–94 CrossRef CAS PubMed.
- D. K. Roper, W. Ahn and M. Hoepfner, J. Phys. Chem. C, 2007, 111, 3636–3641 CAS.
- J. R. Cole, N. A. Mirin, M. W. Knight, G. P. Goodrich and N. J. Halas, J. Phys. Chem. C, 2009, 113, 12090–12094 CAS.
- A. S. Watson, M. Mortensen and A. K. Simon, Cell Cycle, 2011, 10, 1719–1725 CrossRef CAS.
- Z. M. Markovic, L. M. Harhaji-Trajkovic, B. M. Todorovic-Markovic, D. P. Kepic, K. M. Arsikin, S. P. Jovanovic, A. C. Pantovic, M. D. Dramicanin and V. S. Trajkovic, Biomaterials, 2011, 32, 1121–1129 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13427c |
| This journal is © The Royal Society of Chemistry 2015 |