A continuous-flow synthesis of 1,4-benzodiazepin-5-ones, privileged scaffolds for drug discovery†
Abstract
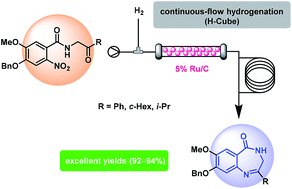
An efficient and gram-scale continuous-flow protocol for the synthesis of the privileged structure 3,4-dihydro-5H-benzo[e][1,4]diazepin-5-one is reported. If compared to the traditional metal mediated non-catalytic reduction procedure, this approach is high yielding and does not require purification steps and therefore could be conveniently used for the generation of compound libraries for drug discovery.

 Please wait while we load your content...
Please wait while we load your content...