Luminescent CdTe quantum dots incarcerated in a columnar matrix of discotic liquid crystals for optoelectronic applications†
Abstract
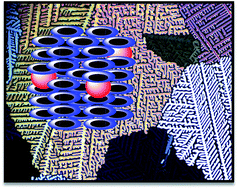
Here in we demonstrate, for the first time, the effects of highly luminescent alkylamine-capped semiconductor cadmium telluride quantum dot (CdTe QD) dispersion on the optical, electrical, and thermal properties and supramolecular order of a discotic liquid crystal (DLC). The insertion and properties of CdTe quantum dots in the columnar mesophase were studied by UV-vis spectroscopy, photoluminescence spectroscopy, polarized optical microscopy, differential scanning calorimetry, X-ray diffraction and DC conductivity. Results indicate uniform dispersion of CdTe QDs in a columnar matrix without disrupting the mesophase but enhancing the conductivity of the system significantly.

 Please wait while we load your content...
Please wait while we load your content...