Preparation of multi-shell structured fluorescent composite nanoparticles for ultrasensitive human procalcitonin detection†
Yue Zhaoa,
Changhua Zhou*ac,
Ruili Wuac,
Lin Lib,
Huaibin Shenac and
Lin Song Li*ac
aKey Laboratory for Special Functional Materials of the Ministry of Education, Henan University, Kaifeng, 475004, P. R. China. E-mail: changhua@henu.edu.cn; lsli@henu.edu.cn; Fax: +86-371-23881358; Tel: +86-371-23881358
bAutobio Diagnostics Co., Ltd, Zhengzhou, 450016, P. R. China
cCollaborative Innovation Center of Nano Functional Materials and Applications, Henan Province, P. R. China
First published on 12th December 2014
Abstract
In this paper, we reported the preparation of carboxyl functionalized quantum dots (QDs)-embedded silica nanoparticles by combining layer-by-layer (LbL) self-assembly technique and a multi-layer protection method. Preformed SiO2 nanoparticles synthesized via the Stöber method were first alternately deposited with positively charged polyelectrolyte (poly(diallyl dimethylammonium chloride), PDDA) and negatively charged CdSe/ZnS QDs by LBL method and further coated by a silica shell. Then, the functionalization of SiO2@PDDA@QDs@SiO2 nanoparticles with hydrophobic hydrocarbons and biocompatible oligomers impart optical imaging properties and good colloidal stability in aqueous solutions at a wide range of pH, PBS buffer, and under thermal treatment. In addition, these newly developed multi-shell structured nanoparticles coupled with specific antibodies were successfully applied in detecting procalcitonin (PCT) antigens using fluorescent lateral flow immunoassay (LFIA). The naked-eye detection limit of fluorescence nanoparticles could achieve 0.1 ng mL−1 of PCT antigens, which was about two hundred times higher than that of colloidal gold-based method.
1. Introduction
Multiplex and high-throughput assays are becoming an essential platform for biomedical imaging, diagnostics, therapy and so on.1–4 In all these areas, considerable attention has focused on the development of fluorescence-encoded multiplex technology as fluorescent labels.5 So far, both organic and inorganic quantum dots (QDs) were widely used as fluorescent labels. Compared to organic fluorophores, QDs have their outstanding photophysical properties such as narrow and size-tunable emission spectra, a robust signal intensity, a broad excitation band, and high photochemical stability.6–9 Especially, with respect to bioapplications, they provide unrivalled cellular imaging and therapeutic detection capabilities.10 However, in practical applications, separation and stability of QDs is difficult to control and deal with, remaining an issue to be solved in the field of biotechnological applications. A promising way to solve these problems is the incorporation of QDs into substrates.6–14 Recently, QDs could be incorporated into microbeads during synthesis, entrapped by solvent swelling or deposited by a layer-by-layer (LbL) adsorption technique.15–18 Compared with the above two methods, polyelectrolyte multilayer microspheres prepared by the LbL technique have advantages of its simplicity, well-controlled size and shape, finely tunable multilayer thickness, variable compositions and functions.19 The LbL-assembled films of negatively charged colloidal SiO2 nanoparticles and a suitable polycation was first reported in detail by Cebeci et al.20 Gao and co-workers successfully obtained multiplexed optical encoding microbeads by incorporating water-soluble CdTe QDs into polystyrene microspheres. Wang, Gao, and colleagues used LbL technique to incorporate QDs into poly(N-isopropylacrylamide) (PNIPAM) hydrogel spheres.21,22 Among of these supports, silica is one of the most used materials in nanotechnology, which provides a stable and accessible surface for immobilizing a variety of functional molecules and small particles.23,24 Besides optical coding methods, surface functional groups and stability are also vital to the applications of QD-encoded beads. Therefore, exploring an efficient route for building up functional nanoprobes with both stable fluorescence and a modifiable surface is still in urgent demand for biological applications.Herein, we reported the preparation of carboxyl functionalized QD-embedded silica nanoparticles by combining layer-by-layer (LbL) self-assembly technique and a multi-layer protection method. First, the obtained negatively charged silica spheres were ready as templates for the subsequent preparation.25 The positively charged polyelectrolyte (poly(diallyldimethylammonium chloride), PDDA) and polymaleic acid n-hexadecanol ester (PMAH) functionalized CdSe/ZnS QDs (QDs–PMAH) were alternately adsorbed onto the silica spheres. Finally, multi-layer protection from silica shell and amphiphilic polymer layers was introduced on SiO2@PDDA@QDs nanoparticles.26 The resulting SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles showed excellent stability (pH, PBS buffer, and under thermal treatment) in aqueous solution. In addition, we attempted to use the obtained multi-shell structured fluorescent nanoparticles to detect procalcitonin (PCT) antigen based on a lateral flow immunoassay (LFIA) biosensor system.
2. Experimental
2.1 Materials
All reagents were used as received without further experimental purification. Cadmium oxide (CdO, 99.99%), sulfur (S, 99.98%), 1-octadecene (ODE, 90%), oleic acid (OA, 90%), selenium (Se, 99.99%), zinc oxide (ZnO, 99.99%), trimethoxy(octadecyl)silane (OTMS), and poly(diallyl dimethylammonium chloride) (PDDA, 20%) were purchased from Aldrich. N-Hexane (95%), n-butanone (99.5%), methanol (99.5%), ethanol (analytical grade), maleic anhydride (MA, 99.5%), acetone (99.5%), benzoyl peroxide (BPO, 98.0%), methylbenzene (99.5%) were obtained from Kermel. Sodium chloride (NaCl), hydrochloric acid (HCl), and sodium hydroxide (NaOH) were all of analytical grade and obtained from Sagon Biotech Co., Ltd. Acetic acid, tetraethyl orthosilicate (TEOS), and ammonia (28%) were obtained from Beijing Chemical Reagent Ltd., China. Deionized water was used for all experiments.2.2 Apparatus
The size and morphology of the nanoparticles were investigated using a JEM 100CX-II transmission electron microscope (TEM) at 100 kV. Room-temperature photoluminescence (PL) spectra were measured with an Ocean Optics spectrophotometer (mode PC2000-ISA) with wavelength ranging from 300 to 800 nm. PL spectrum was taken using an excitation wavelength of 365 nm. The hydrodynamic sizes and zeta potential of PL nanoparticles were obtained from a dynamic light scattering (DLS, Malvern Instruments Corporation, Nano ZS) at 25 °C. X-ray diffraction (XRD) studies of nanoparticles were carried out with a Philips X'Pert Pro X-ray diffractometer using Cu Ka radiation.2.3 Synthesis of sandwich-like structured SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min). The negatively charged (carboxyl groups modified) QDs could be further operated by LbL self-assembly technique.
000 rpm for 30 min). The negatively charged (carboxyl groups modified) QDs could be further operated by LbL self-assembly technique.3. Results and discussion
3.1. Synthesis of SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles
The fabrication flow of the proposed, highly bright QDs-embedded silica nanoparticles with uniform size and composition by the LbL self-assembly technique and a multi-layers protection method is illustrated in Scheme 1. Silica spheres could be created through the classical Stöber method, which usually relies on sol–gel chemistry involving the hydrolysis of TEOS in an alcohol/water solution using ammonia as the catalyst.33 The LbL assembly approach require water-soluble QDs, the as-prepared hydrophobic CdSe/ZnS QDs could be water solubilized by using a PMAH-mediated encapsulation method as reported previously. In addition, the pH value of solution is also highlighted as an important factor to achieve sufficient QD surface coverage. We optimized the process of synthesis at the correct pH conditions, resulting in a higher QD coverage after three wash cycles. First, negatively charged SiO2 particles were reversed the surface charge by adsorbing positively charged PDDA at pH 5.0. Then, SiO2@PDDA@QDs particles were prepared by linking the positive charged SiO2@PDDA and negatively charged QDs at pH value of 9.0 through electrostatic interaction. Afterwards, another silica protecting layer was coated on the SiO2@PDDA@QDs to prevent the QDS from off the SiO2@PDDA nanoparticles and improve the stability in harsh environments. To satisfy the requirement of biocompatibility, the surfaces of the SiO2@PDDA@QDs@SiO2 nanoparticles were further passivated using OTMS, which changed the SiO2@PDDA@QDs@SiO2 from hydrophilic to hydrophobic and provided an additional protection. Afterwards, the hydrophobic SiO2@PDDA@QDs@SiO2@OTMS nanoparticles were converted back to water-soluble again by grafting the hydrophobic surface with PMAH. The obtained carboxylic-functionalized nanoparticles could enable further conjugation of targeting biomolecules. | ||
| Scheme 1 Schematic illustration of a multi-shell protecting procedure for the synthesis of SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles. | ||
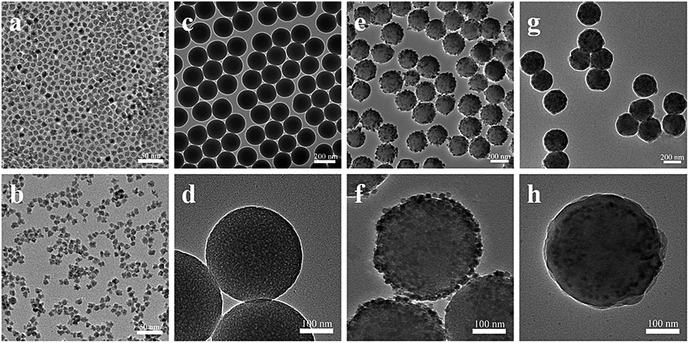
The morphology and size of CdSe/ZnS QDs before (a) and after (b) phase transfer were characterized by TEM and shown in Fig. 1. The hydrophobic QDs were nearly monodisperse with an average diameter of 9.8 nm in CHCl3. After phase transfer, the hydrophilic QDs in water still remained monodisperse in size and no obvious shape change and aggregation were observed. Fig. 1(c) showed the TEM image of silica nanoparticles with an average diameter of about 220 nm. A closer TEM image of a single nanoparticle was demonstrated in Fig. 1(d), which clearly indicated silica nanoparticle surfaces were smooth. The morphology and size of SiO2@PDDA@QDs were characterized by TEM in Fig. 1(e). It is clearly showed that their surfaces were evenly covered by QDs, and the QD-doped silica nanoparticle surfaces were comparatively rough. A closer image of a single particle was demonstrated in Fig. 1(f), which indicated the number of QDs on the SiO2@PDDA was estimated to be ≈400–900 per single PDDA-coated SiO2 nanoparticle. TEM image (Fig. 1(g)) exhibited the efficacy of the Stöber method in encapsulating the SiO2@PDDA@QDs nanoparticles within silica. All the samples of silica-coated SiO2@PDDA@QDs nanoparticles appeared to be uniform in size and well dispersed. From the zoomed image of Fig. 1(h), a single SiO2@PDDA@QDs nanoparticle with a 15 nm thick silica layer could be clearly seen. By controlling the concentration of SiO2@PDDA@QDs nanoparticles and the hydrolysis of TEOS, the SiO2@PDDA@QDs nanoparticles with tunable silica shells could be prepared.34 In our study, the silica shell thickness could be tuned in the range of 15–30 nm (Fig. S2†). After further surface modification with OTMS and PMAH, these layers are not visible under TEM because the organic molecules are not electron-dense materials. Apparently, the multistep modification processes did not cause the aggregation of nanoparticles, which was also confirmed by dynamic light scattering (DLS) measurement in an aqueous environment.
The hydrodynamic size distribution changes in the preparation of SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles could be monitored in real time by dynamic light scattering (DLS). Beyond a dimensional change, the dispersion of the nanospheres was observed in solution.35 As shown in Fig. 2(a) of the information, the hydrodynamic diameters (HD) of SiO2, SiO2@PDDA, SiO2@PDDA@QDs and SiO2@PDDA@QDs@SiO2 were 245.9, 316.4, 305.1 and 272.3 nm, respectively, which were larger than the measured diameters from the TEM images. The DLS result showed a HD of 396.1 nm for SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles, which was much larger than the nanoparticles produced in the process of the former. This is because PMAH provided plentiful carboxylic acid groups on nanoparticles surface in order to carry with the high negative charges in water. This negative surface charge not only increased the colloidal HD compared to the natural size, but also created an electrical double layer in aqueous solution to prevent QDs from aggregation.
 | ||
| Fig. 2 (a) Comparing the DLS data of nanoparticles at different stages; (b) zeta potential changes in the preparation of SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles. | ||
Furthermore, zeta-potential measurements were used as a second independent method to confirm multilayer formation (Fig. 2(b)). The zeta potential of silica nanoparticles showed a negatively surface charge of −47.4 mV. The presence of PDDA, which is cationic, caused a reversal in zeta potential to 48.2 mV. Subsequent deposition of PMAH coated QDs onto the SiO2@PDDA nanoparticles made surface charge neutralization to a value of −25.6 mV. SiO2@PDDA@QDs particles were encapsulated by SiO2 shell, and zeta potential measured in water is −42.0 mV. The carboxylic functionalized SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles added up to −49.0 mV. Multilayer coated silica particles were demonstrated by the alternating sign of the surface electric potential.
To further prove the growth of CdSe/ZnS QDs shell, the X-ray diffraction (XRD) pattern was used to indicate the existence of QDs in SiO2@PDDA@QDs composites. Fig. 3 displayed the XRD pattern of SiO2 and corresponding SiO2@PDDA@QDs nanoparticles. At 2θ values of 22.45°, there appeared a wide peak in SiO2 and SiO2@PDDA@QDs particles respectively, which can be indexed to SiO2 spheres. In the case of SiO2@PDDA@QDs, three obvious XRD diffraction peaks are located at 26.45°, 43.83°, and 52.50° corresponding to (111), (220), and (311) planes of the CdSe/ZnS QDs. So CdSe/ZnS QDs were coated successfully on the surface of SiO2@PDDA@QDs nanoparticles.
PL study is a powerful tool to investigate the optical properties of these novel fluorescent nanoparticles. During different preparation stages, the changes of emission peak and intensity were shown in Fig. 4. The PL spectrum of the hydrophobic CdSe/ZnS QDs in CHCl3 has a maximum peak at 610 nm and narrow FWHM with 34 nm. CdSe/ZnS QDs in different preparation stages exhibited a slightly red-shifted (<2 nm) emission peak from that of the original hydrophobic QDs (Fig. 4(a)). And the peak width of CdSe/ZnS QDs were the same as before encapsulation. At the meantime, PL intensity of original CdSe/ZnS QDs was slightly reduced (about 14%) after the transfer, adsorption, and coating process (Fig. 4(b)). These results indicated that CdSe/ZnS QDs were assembled on SiO2 particle surface without PL changes associated with agglomeration, ripening, or changes in refractive index at the QD surface.
3.2. Stability tests of aqueous SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles in physiological conditions
The stability and optical properties of SiO2@PDDA@QDs@SiO2@OTMS@PMAH under various biologically relevant conditions of pH, temperature, and PBS buffer were further examined (Fig. 5). Previously a number of groups have built QD-based pH sensors because QDs are sensitive to chemicals in the surrounding environment such as acids, bases, ions, and proteins. For example, pH values can be directly correlated with QD PL intensity because QD fluorescence, in general, is quenched in acids and is enhanced in bases. Next, we systematically studied the influence of pH values on stability of SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles. The pH value of the SiO2@PDDA@QDs@SiO2@OTMS@PMAH solution was varied by dropwise addition of HCl or NaOH. Remarkably, the nanoparticles preserved high photostability and strong PL in the wide pH range 6–12. Such great improvement of pH stability was attributable to the distinctive surface properties of the silica and amphiphilic polymer multilayer shell. In addition, the PL intensity of our SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles changed by less than 55% at pH 1 and 14 after 1 h incubation. | ||
| Fig. 5 pH (a), temperature (b) and PBS buffer (c) stability comparison of SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles. The solution was incubated for 1 h before the pH and temperature stability test. | ||
Temperature is another important parameter for biological experiments. Fig. 5(b) showed the variation of PL intensity of SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles after heating. Although there was clear temperature dependence, the nanoparticles retained about 70% of their initial PL intensity even after being heated to 80 °C. Over the temperature range of most relevance to biological experiments (20 °C to 40 °C), the PL intensity varied by less than 15%.
These nanoparticles also exhibited stable PL when dispersed in common buffers like PBS buffer (pH = 7.2). As shown in Fig. 5(c), the PL intensity of SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles only decreased slightly (<5%) and became constant after 600 h. These data suggest the potential of these nanoparticles as robust biomarkers due to their stronger fluorescence and better stability.
3.3. Demonstration of the SiO2@PDDA@QDs@SiO2@OTMS@PMAH nanoparticles as fluorescence labels for detecting PCT antigen
Human procalcitonin (PCT) is a biomarker that reacts with high sensitivity and specificity to generalized infection and sepsis.36,37 It correlates better than all other existing parameters to the inflammatory activity of the immune system and can be used to diagnose and to monitor severe bacterial infections and sepsis. PCT supports early diagnosis and clinical decision making which could direct an effective therapy at the right time and avoid unnecessary spending for critically ill patients. Therefore, it is necessary to develop new high sensitivity detection method to detect PCT.To explore the feasibility of composite nanoparticles in PCT antigen detection, we conjugated the obtained high fluorescent nanoparticles with PCT antibody using standard carbodiimide cross-linking reaction and designed an immuno-sandwich assay for PCT antigen detection. During the assay (as shown in Scheme 2),38 aqueous sample was applied to the sample pad and transported to the conjugation zone, where the preabsorbed and dried composite-antibodies were rehydrated and washed off and composite-antibody were formed. The sample further migrated to the test line where the second antibodies were immobilized, leading to the formation of immunosandwich conjugates according to the specific antigen–antibody binding on the test line, whereas excess composite-antibody further migrated toward the absorption pad. The selectivity of the functional nanoparticle depends on the antibody coupled with nanoparticles. In our study, the high specificity of anti-PCT monoclonal antibody could offer the high selectivity to the functional nanoparticle. The fluorescence signal of composite nanoparticles on the test line was observed by the fluorescence detector, which has a lightening of 365 nm LED lamp, at 15 min after the addition of analytes. Quantitative detection was realized by recording the PL intensity of nanoparticles captured on the test line. Before use, the PCT standard was diluted into a series of the concentration of the solution. The fluorescence signals were recorded by test strip reader and digital camera. The resulting calibration plots were linear as shown in Fig. 6(a); the correlation coefficients was 0.9788. The corresponding calibration plot of response versus PCT concentration was linear over the 0–10 ng mL−1 range and was suitable for quantitative analysis. Meanwhile, fluorescence images were also easily observed as shown in Fig. 6(b). We observed no signal in blank test, while proportional changed in fluorescence brightness of the test lines associated with the concentration of PCT. Such an observation was expected because the test line captures more composite-antibody when the analyte concentration was higher. The increased fluorescent signal strength at different PCT concentrations allowed us to study on rapid and quantitative analysis for PCT for clinical diagnosis. Furthermore, a red signal band from the target concentration as low as 0.1 ng mL−1 could be easily seen even with visual inspection. Over all, the achieved composite nanoparticles in this study were demonstrated to be a sensitive and reliable label material for such a bioassay.
 | ||
| Fig. 6 QDs-based LFIA linear response (a) and typical photo image of detection results by the fluorescent strip under the ultraviolet irradiation (b). | ||
4. Conclusions
In conclusion, using the LbL self-assembly technique and a multi-layer protection method, biocompatible SiO2@PDDA@QDs@SiO2@OTMS@PMAH composite nanoparticles were successfully prepared, and the material exhibited a high fluorescence intensity and good colloidal stability. We also confirmed the suitability of achieved nanoparticles for in vitro use by monitoring the highly amplified fluorescence signals from nanoparticles-labelled PCT. Further development and optimization of this technology should offer new opportunities to fundamental biophysics as well as clinical diagnostics.Acknowledgements
The authors gratefully acknowledge the support of the National Natural Science Foundation of China (no. 21201055 and no. 61474037), Program for Science & Technology Innovation Talents in Universities of Henan Province (no. 14HASTIT009), Basic and cutting-edge technology research projects of Henan Province (no. 142300413207), and Program for Changjiang Scholars and Innovative Research Team in University (no. PCS IRT1126).Notes and references
- R. Walt, Science, 2000, 287, 451–452 CrossRef.
- W. Ma, H. T. Liu, X. P. He, Y. Zang, J. Li, G. R. Chen, H. Tian and Y. T. Long, Anal. Chem., 2014, 86, 5502–5507 CrossRef CAS PubMed.
- H. L. Zhang, X. L. Wei, Y. Zang, J. Y. Cao, S. Liu, X. P. He, Q. Chen, Y. T. Long, J. Li, G. R. Chen and K. Chen, Adv. Mater., 2013, 25, 4097–4101 CrossRef CAS PubMed.
- Y. Zhang, J. Qian, D. Wang, Y. Wang and S. He, Angew. Chem., Int. Ed., 2013, 52, 1148–1151 CrossRef CAS PubMed.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Nat. Methods, 2008, 5, 763–775 CrossRef CAS PubMed.
- L. Wang, W. Chen, W. Ma, L. Liu, W. Ma, Y. Zhao, Y. Zhu, L. Xu, H. Kuang and C. Xu, Chem. Commun., 2011, 47, 1574–1576 RSC.
- X. L. Sun, B. Zhu, D. K. Ji, Q. B. Chen, X. P. He, G. R. Chen and T. D. James, ACS Appl. Mater. Interfaces, 2014, 6, 10078–10082 CAS.
- X. P. He, Q. Deng, L. Cai, C. Z. Wang, Y. Zang, J. Li, G. R. Chen and H. Tian, ACS Appl. Mater. Interfaces, 2014, 6, 5379–5382 CAS.
- Y. Zhu, Z. Li, M. Chen, H. M. Cooper and Z. P. Xu, Chem. Mater., 2012, 24, 421–423 CrossRef CAS.
- B.-H. Jun, D. W. Hwang, H. S. Jung, J. Jang, H. Kim, H. Kang, T. Kang, S. Kyeong, H. Lee, D. H. Jeong, K. W. Kang, H. Youn, D. S. Lee and Y.-S. Lee, Adv. Funct. Mater., 2012, 22, 1843–1849 CrossRef CAS.
- X. P. He, R. H. Li, S. Maisonneuve, Y. Ruan, G. R. Chen and J. Xie, Chem. Commun., 2014, 50, 14141–14144 RSC.
- Q. Ma, I. C. Serrano and E. Palomares, Chem. Commun., 2011, 47, 7071–7073 RSC.
- Y. He, Y. Su, X. Yang, Z. Kang, T. Xu, R. Zhang and S. T. Lee, J. Am. Chem. Soc., 2009, 131, 4434–4438 CrossRef CAS PubMed.
- B. H. Jun, G. Kim, M. S. Noh, H. Kang, Y. K. Kim, M. H. Cho, D. H. Jeong and Y. S. Lee, Nanomedicine, 2011, 6, 1463–1480 CrossRef CAS PubMed.
- T. Song, Q. Zhang, C. Lu, X. Gong, Q. Yang, Y. Li, J. Liu and J. Chang, J. Mater. Chem., 2011, 21, 2169–2177 RSC.
- A. T. Nagaraja, A. Sooresh, K. E. Meissner and M. J. McShane, ACS Nano, 2013, 7, 6194–6202 CrossRef CAS PubMed.
- S. Beyer, W. C. Mak and D. Trau, Langmuir, 2007, 23, 8827–8832 CrossRef CAS PubMed.
- Y. Chen, B. Jiang, Y. Xiang, Y. Chai and R. Yuan, Chem. Commun., 2011, 47, 7758–7760 RSC.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- F. C. Cebeci, Z. Wu, L. Zhai, R. E. Cohen and M. F. Rubner, Langmuir, 2006, 22, 2856–2862 CrossRef CAS PubMed.
- M. Kuang, D. Wang, H. Bao, M. Y. Gao and M. Jiang, Adv. Mater., 2005, 17, 267–270 CrossRef CAS.
- Y. Gong, M. Y. Gao, D. Wang and H. Mohwald, Chem. Mater., 2005, 17, 2648–2653 CrossRef CAS.
- F. Erogbogbo, K.-T. Yong, I. Roy, G. Xu, P. N. Prasad and M. T. Swihart, ACS Nano, 2008, 2, 873–878 CrossRef CAS PubMed.
- J.-H. Park, L. Gu, G. Maltzahn, E. Ruoslahti, S. N. Bhatia and M. J. Sailo, Nat. Mater., 2009, 8, 331–336 CrossRef CAS PubMed.
- R. Gui and X. An, RSC Adv., 2013, 3, 20959–20969 RSC.
- B. Zhang, R. Hu, Y. Wang, C. Yang, X. Liu and K.-T. Yong, RSC Adv., 2014, 4, 13805–13816 RSC.
- H. Shen, H. Wang, Z. Tang, J. Z. Niu, S. Lou, Z. Du and L. S. Li, CrystEngComm, 2009, 11, 1733–1738 RSC.
- J. Riegler and T. Nann, Anal. Bioanal. Chem., 2004, 379, 913–919 CrossRef CAS PubMed.
- C. Zhou, H. Yuan, X. Wang, H. Shen, Y. Guo, L. Xu, F. Wu, L. Ma and L. S. Li, Sci. Adv. Mater., 2013, 5, 285–294 CrossRef CAS PubMed.
- G. H. Bogush, M. A. Tracy and C. F. Zukoski IV, J. Non-Cryst. Solids, 1988, 104, 95–106 CrossRef CAS.
- R. Ashayer, M. Green and S. H. Mannan, J. Nanopart. Res., 2010, 12, 1489–1494 CrossRef CAS.
- N. Insin, J. B. Tracy, H. Lee, J. P. Zimmer, R. M. Westervelt and M. G. Bawendi, ACS Nano, 2008, 2, 197–202 CrossRef CAS PubMed.
- A. H. Lu, G. P. Hao and Q. Sun, Angew. Chem., Int. Ed., 2011, 50, 9023–9025 CrossRef CAS PubMed.
- P. H. Zhang, J. T. Cao, Q. H. Min and J. J. Zhu, ACS Appl. Mater. Interfaces, 2013, 5, 7417–7424 CAS.
- Z. Popović, W. Liu, V. P. Chauhan, J. Lee, C. Wong, A. B. Greytak, N. Insin, D. G. Nocera, D. Fukumura, R. K. Jain and M. G. Bawendi, Angew. Chem., 2010, 122, 8831–8834 CrossRef.
- S. Liaudat, E. Dayer, G. Praz, J. Bille and N. Troillet, Eur. J. Clin. Microbiol., 2001, 20, 524–527 CrossRef CAS.
- M. Oppert, A. Reinicke, C. Müller, D. Barckow, U. Frei and K.-U. Eckardt, Resuscitation, 2002, 53, 167–170 CrossRef CAS.
- Z. Jin and N. Hildebrandt, Trends Biotechnol., 2012, 30, 394–403 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13362e |
| This journal is © The Royal Society of Chemistry 2015 |