Graphene oxide–TiO2 composite as a novel adsorbent for the preconcentration of heavy metals and rare earth elements in environmental samples followed by on-line inductively coupled plasma optical emission spectrometry detection†
Yanan Zhang,
Cheng Zhong,
Qiangying Zhang,
Beibei Chen,
Man He* and
Bin Hu
Key Laboratory of Analytical Chemistry for Biology and Medicine (Ministry of Education), Department of Chemistry, Wuhan University, Wuhan 430072, China. E-mail: heman@whu.edu.cn; Fax: +86 27 68754067; Tel: +86 27 87653132
First published on 15th December 2014
Abstract
A graphene oxide (GO)–titanium dioxide (TiO2) composite was prepared by hydrolysis of Ti(BuO)4 to TiO2 on GO nanosheets. The mass ratio of GO–TiO2 in the composite was optimized and the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite was selected as the adsorbent for the preconcentration of heavy metals and rare earth elements (REEs). The prepared GO–TiO2 (1
1) composite was selected as the adsorbent for the preconcentration of heavy metals and rare earth elements (REEs). The prepared GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite was characterized by Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis and differential thermal gravimetry measurements (TGA/DTG), powder X-ray diffraction (XRD) and transmission electron microscopy (TEM). The results indicated that the anchored TiO2 was in the nanometer-sized anatase form and the GO–TiO2 (1
1) composite was characterized by Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis and differential thermal gravimetry measurements (TGA/DTG), powder X-ray diffraction (XRD) and transmission electron microscopy (TEM). The results indicated that the anchored TiO2 was in the nanometer-sized anatase form and the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite inherited both features of GO and TiO2. It exhibited good stability, strong anti-interference capability, and high adsorption capacities for target ions. By using GO–TiO2 (1
1) composite inherited both features of GO and TiO2. It exhibited good stability, strong anti-interference capability, and high adsorption capacities for target ions. By using GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite as the micro-column packing material, a method for on-line solid phase extraction (SPE) coupling with inductively coupled plasma optical emission spectrometry (ICP-OES) was developed for the quantification of heavy metals and REEs in environmental water and sediment samples. The parameters influencing the on-line SPE of target elements including pH, elution conditions, sample flow rate and sample volume were optimized. Under the optimized conditions, a wide linear range over 0.5–1000 ng mL−1 was obtained for target elements. The detection limits (LODs) and the relative standard deviations (RSDs) of Cu, Pb, La, Ce, Eu, Dy and Yb were 0.48, 2.64, 0.41, 0.24, 0.13, 0.26 and 0.21 ng mL−1 and 6.4, 9.8, 8.6, 3.2, 5.6, 4.5 and 6.2% (C = 10 ng mL−1, n = 7), respectively. For all the analytes, the overall adsorption process was controlled by diffusion and chemical reaction, and chemical reaction played a dominant role. The established method of on-line SPE-ICP-OES was validated by analyzing Certified Reference Material GBW07301a (Stream sediment), and the determined values are in good agreement with the certified values. The method was also applied for the determination of the target heavy metals and REEs in environmental water (even sea water) and sediment samples, and the recoveries were 82.4–115.5% and 83.8–114.8% for the spiked water and sediment samples, respectively.
1) composite as the micro-column packing material, a method for on-line solid phase extraction (SPE) coupling with inductively coupled plasma optical emission spectrometry (ICP-OES) was developed for the quantification of heavy metals and REEs in environmental water and sediment samples. The parameters influencing the on-line SPE of target elements including pH, elution conditions, sample flow rate and sample volume were optimized. Under the optimized conditions, a wide linear range over 0.5–1000 ng mL−1 was obtained for target elements. The detection limits (LODs) and the relative standard deviations (RSDs) of Cu, Pb, La, Ce, Eu, Dy and Yb were 0.48, 2.64, 0.41, 0.24, 0.13, 0.26 and 0.21 ng mL−1 and 6.4, 9.8, 8.6, 3.2, 5.6, 4.5 and 6.2% (C = 10 ng mL−1, n = 7), respectively. For all the analytes, the overall adsorption process was controlled by diffusion and chemical reaction, and chemical reaction played a dominant role. The established method of on-line SPE-ICP-OES was validated by analyzing Certified Reference Material GBW07301a (Stream sediment), and the determined values are in good agreement with the certified values. The method was also applied for the determination of the target heavy metals and REEs in environmental water (even sea water) and sediment samples, and the recoveries were 82.4–115.5% and 83.8–114.8% for the spiked water and sediment samples, respectively.
1 Introduction
Heavy metal pollution represents a serious threat to the ecological environment and especially to human beings due to their gradual accumulation in the environment and toxicological effects on living organisms.1–3 Copper is an essential substance to human life, but in high doses it can cause anemia, liver and kidney damage as well as stomach and intestinal irritation.4–6 Typical symptoms of lead poisoning include abdominal pain, anemia, headaches and convulsions, chronic nephritis of the kidney, brain damage and central nervous-system disorders.7 Rare earth elements (REEs) can enter the human body via air, food and drinking water, as well as therapeutic and diagnostic agents.8 Long-time exposure to REEs may exert a harmful influence on human liver, kidney, lung, and bone quality and result in some negative effects to immunologic system.8–10 Consequently, the development of effective methods for determination of heavy metals and REEs in environmental samples is of significant importance.Inductively coupled plasma optical emission spectrometry (ICP-OES) has been widely applied to determine trace metals due to its special features such as multi-element analysis ability, relatively high sensitivity and wide linear range. However, direct determination of trace metals by ICP-OES is often difficult due to the low concentration of target metals and the complexity of sample. Thus, separation/preconcentration of trace metals is usually needed prior to instrumental measurement.11–15
Solid phase extraction (SPE) has become one of the most widely used sample pretreatment techniques for the analysis of heavy metals or REEs, due to its simple operation, high enrichment factor, rapid phase separation, and the ability to combine with different detection techniques.13 The on-line micro-column SPE technique based on flow injection (FI) has the advantages of reducing the loss of analyte, high sample throughput, and low contamination risk. The adsorbent packed in the micro-column plays a significant role in the FI on-line SPE system. The ideal adsorbent should possess certain properties such as low backpressure in the column, appropriate affinity and satisfactory selectivity for interest analytes. Carbon-based materials have shown unique advantages as SPE adsorbents, such as high adsorption capacity, favorable chemical and thermal stability and cost-effectiveness. As the representatives of carbon-based materials, fullerenes,16 carbon nanofibers (CNFs),17 single-wall carbon nanotubes (SWCNTs),18 multi-wall carbon nanotubes (MWCNTs)19,20 and graphene21,22 have been investigated as SPE adsorbents for the separation and preconcentration of metal ions.
As a novel type of carbon-based materials, graphene oxide (GO) has been regarded as a potential adsorbent for the preconcentration of trace metal ions due to its special features. GO maintains the basic framework of graphene which has a large surface area (theoretical value of 2630 m2 g−1).23 Both sides of the planar sheets of graphene are available for adsorption of analytes compared with fullerenes and CNTs. In addition, various oxygen-containing groups (e.g., carboxyl, hydroxyl and epoxy) on GO offer more binding sites than graphene for adsorption of metal ions and make it easily functionalized. These features are responsible for its high adsorption capacity and high chemical activity. Recently, the application of GO as a sorbent for the removal of metal ions from aqueous solutions has been reported.24–27 Rafal Sitko et al.26 demonstrated that the maximum adsorption capacities of Cu(II), Zn(II), Cd(II) and Pb(II) on GO were 294, 345, 530, 1119 mg g−1, respectively, and the affinity in the competitive adsorption experiments followed the order of Pb(II) > Cu(II) ≫ Cd(II) > Zn(II). The interaction between Eu(III) and GO was investigated by Wang's group and the adsorption capacity of Eu(III) on GO was as high as 175.4 mg g−1.27 The utilization of GO or modified GO as the adsorbent for the separation/preconcentration of trace metal ions has also been reported.28–30 Deng et al.28 took the advantages of the hydrophilic property of GO and developed a cloud point extraction-like methodology to determine metal ions. Su et al.29 prepared a magnetic Fe3O4@SiO2@polyaniline–GO composite and used it for the preconcentration of REEs. Wang et al.30 prepared SmtA–GO@cytopore adsorbent and successfully used it in on-line SPE for highly selective adsorption and preconcentration of ultra-trace cadmium. Besides, GO can be easily prepared from graphite,31,32 which is cheaper in comparison with commercially available SPE materials. All these advantages make GO or functionalized GO more attractive as a SPE adsorbent for the adsorption of heavy metals and REEs.
Despite the impressive properties of GO, direct application of GO as on-line SPE adsorbent is still an insurmountable challenge. The high hydrophilicity and good dispersibility of GO in the aqueous medium can cause adsorbent loss and high pressure in the SPE column operation. In order to resolve the above problem, modification of inorganic materials onto the GO sheets proves to be a feasible strategy.33,34
Titania (TiO2) is an amphoteric metal oxide containing two types of hydroxyl groups on the surface and has high chemical stability over a wide pH range.35,36 Due to the excellent properties of TiO2, it has been successfully applied to retain heavy metals or REEs in on-line SPE.11–15,37 The direct application of GO as on-line SPE adsorbent was difficult and this work aims to prepare a GO–TiO2 composite as the micro-column packing material which not only reduces the solubility of GO but also inherits both advantages of GO and TiO2. And for the first time, the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite was used as the on-line micro-column packing material for separation and preconcentration of Cu(II), Pb(II), La(III), Ce(III), Eu(III), Dy(III) and Yb(III) in environmental water and sediment samples which broadened the application of GO in SPE of metal ions.
1) composite was used as the on-line micro-column packing material for separation and preconcentration of Cu(II), Pb(II), La(III), Ce(III), Eu(III), Dy(III) and Yb(III) in environmental water and sediment samples which broadened the application of GO in SPE of metal ions.
2 Experimental
2.1 Apparatus
ICP-OES (Intrepid XSP Radial, Thermo, USA) with a concentric nebulizer and a cinnabar spray chamber was used for the determination. And the operation conditions were presented in Table 1. The pH values were adjusted with a pH meter (320-S, Mettler Toledo Instruments Co. Ltd., Shanghai, China) supplied with a combined electrode. A microwave-accelerated digestion system (WX-4000, Shanghai EU Microwave Chemistry Technology Co. Ltd., Shanghai, China) and SKML model temperature control heating panel (Beijing, China) were used for sample digestion. A digital electric agitator (RW20, IKA, Germany) and a vacuum drying oven (DZG-6020, Senxin Instrument Factory, Shanghai, China) were applied for the preparation of GO, TiO2 and GO–TiO2 composite. An ultrasonic cleaner (KQ 5200DE, Shu mei Instrument Factory, Kun shan, China) was applied to prepare GO dispersion. A flow injection analysis processor (FIA-3110, Titan Instrument Co. Ltd., Beijing, China) and a self-made PTFE micro-column (20 mm × 2.0 mm i.d.) packed with GO–TiO2 composite were used in the on-line separation and preconcentration process.| RF generator power (W) | 1150 | |
| Frequency of RF generator (MHz) | 30.05 | |
| Coolant gas flow rate (L min−1) | 14 | |
| Auxiliary gas (L min−1) | 0.5 | |
| Carrier gas flow rate (L min−1) | 0.5 | |
| Solution uptake rate (mL min−1) | 1.3 | |
| Max integration times (s) | 27 | |
| Analytical wavelength (nm) | Ce 413.380 | La 408.670 |
| Cu 324.754 | Pb 220.353 | |
| Dy 353.170 | Yb 369.419 | |
| Eu 381.967 |
The prepared materials were characterized by a Fourier transform infrared spectrometer (FT-IR, IS10, Thermo Nicolet, USA), a transmission electron microscope (TEM, JEM-2100, JEOL Ltd., Japan), and a powder X-ray diffraction diffractometer (XRD, D8 advance, Bruker, Germany). Thermogravimetric analysis and differential thermal gravimetry measurements (TGA/DTG) were carried out on a Setsys16 (Setaram, France) in N2 atmosphere.
2.2 Standard solutions and reagents
The stock standard solutions (1.000 g L−1) of metal ions (Cu, Pb, La, Ce, Eu, Dy and Yb) were prepared by dissolving certain amounts of CuSO4·5H2O, Pb(NO3)2, Ce(NO3)3·6H2O in 2% HNO3 and specpure oxides of La, Eu, Dy and Yb in 2% HCl. Working standard solutions were prepared daily from the stock standard solutions. High purity water (18.2 MΩ cm) obtained from Milli-Q Element system (Millipore, Molsheim, France) was used throughout the experimental process, and the employed reagents were at least analytical grade.Graphite powder (325 mesh, 99.9995%) was purchased from Alfa Aesar (MA, USA). Phosphorus pentoxide (P2O5), potassium thiosulfate (K2S2O8), potassium permanganate (KMnO4), hydrochloric acid (HCl), sulfuric acid (H2SO4), hydrogen peroxide (H2O2) and ethanol (EtOH) were bought from Sinopharm Chemistry Reagent Co. Ltd. (Shanghai, China). Tetra-n-butyl titanate (Ti(BuO)4) was purchased from Tianjin Kermel Chemical Reagent Co. Ltd. (Tianjin, China).
2.3 Preparation of GO–TiO2 composite
GO was prepared according to Hummers' method31,32 with several modifications. 3 g graphite powder was dispersed into a mixture of 12 mL of concentrated H2SO4 containing 2.5 g of K2S2O8 and 2.5 g of P2O5, and the mixture was heated to 80 °C and kept stirring for 4.5 h in an oil bath. Then, the mixture was diluted with 0.5 L of high purity water and left overnight. After that, the product was obtained by suction filtration and dried at 40 °C in a vacuum oven. The product of preoxidation was put into 120 mL of concentrated H2SO4, followed by gradual addition of 15 g of KMnO4 under stirring in an ice bath, and the temperature was kept below 20 °C. The mixture was stirred at 35 °C for 7 days. Then, 250 mL of water was added and the mixture was stirred for another 2 h at 98 °C. After that, the mixture was diluted with 0.7 L of water. 20 mL of H2O2 (30%, v/v) was added along with bubbling. The suspension was stirred for 2 h and left overnight. The upper solution was decanted and the precipitate was washed with 1.3 L of HCl (10%, v/v) and then washed with water until the supernatant was neutral. After centrifugation, the collected precipitate was freeze-dried and brown product was obtained.The GO–TiO2 composite was in situ synthesized based on Liang's work38 with modifications. The composite was prepared by in situ hydrolysis of Ti(BuO)4 to TiO2 on GO sheets. Briefly, 120 mg of GO was dispersed in a solution of EtOH/H2O (350 mL/25 mL) by ultrasonic treatment for 0.5 h. The GO suspension was heated to 80 °C in an oil bath. Then the Ti(BuO)4 dissolved in EtOH/H2SO4 (25 mL/0.375 mL) was gradually added to the GO suspension. The mixture was kept stirring at 80 °C for 12 h. The obtained suspension was centrifuged and washed with 500 mL water and the final product was dried at 60 °C in a vacuum oven. The composites with different mass ratios of GO to TiO2 were synthesized in the range of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–9
9–9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by varying the initial proportion of GO/Ti(BuO)4. The TiO2 material was synthesized as well under the same conditions without GO addition.
1 by varying the initial proportion of GO/Ti(BuO)4. The TiO2 material was synthesized as well under the same conditions without GO addition.
2.4 Column preparation
Fifty milligrams of GO–TiO2 composite was filled into a PTFE micro-column. Some cotton wool was used on both ends to prevent packing losses. Before use, 0.5 mol L−1 HNO3 and 0.1 mol L−1 NH4Ac were passed sequentially through the column in order to clean and condition the packed material.2.5 Sample preparation
2.6 General SPE procedure
Sample solutions containing Cu, Pb, La, Ce, Eu, Dy and Yb ions were prepared by stepwise dilution of the stock solutions and adjusted to pH 5.0 with 1.0 mol L−1 NaOH and 1.0 mol L−1 HNO3. 7 mL of sample solution was pumped through the micro-column at a flow rate of 2 mL min−1. The adsorbed analytes were eluted with the 0.7 mL of 1.0 mol L−1 HNO3 at a flow rate of 1.2 mL min−1 and determined by ICP-OES. 7 mL of ultrapure water was employed as the blank and subjected to the same procedure.3 Results and discussion
3.1 Optimization of the ratio of GO/TiO2
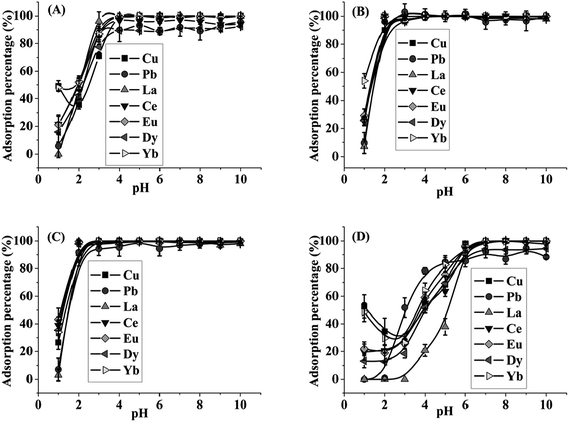
To optimize the ratio of GO/TiO2 in the GO–TiO2 composite, the effects of pH on the retention of target metals on micro-column packed with GO–TiO2 composite in different ratios of GO/TiO2 were studied with 0.5 μg mL−1 Cu, Pb, La, Ce, Eu, Dy and Yb as model elements. The results are shown in Fig. 1. As can be seen, the quantitative adsorption of target ions on TiO2 was achieved in the range of pH 7–10. And the target metals were quantitatively retained on GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) composite at pH 4–10. For GO–TiO2 (1
9) composite at pH 4–10. For GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) composite and GO–TiO2 (1
3) composite and GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite, the target ions could be quantitatively adsorbed at pH 3–10. The edge carboxyl groups of GO are highly protonated at pH 1 (ref. 39) and this may explain the low adsorption amounts of metal ions under this condition. The results indicated that the quantitative retention (>90%) of the target ions at lower pH could be obtained on the adsorbent with a higher ratio of GO, indicating that GO played a key role in retention of the analytes in agreement with the pH-dependent properties of GO which was negatively charged over the pH range from 2 to 10.39,40 And the adsorption mechanism may be mainly due to the binding of metal ions with the negatively charged functional groups available on GO surfaces. It should be noted that GO–TiO2 composite with the ratio of 3
1) composite, the target ions could be quantitatively adsorbed at pH 3–10. The edge carboxyl groups of GO are highly protonated at pH 1 (ref. 39) and this may explain the low adsorption amounts of metal ions under this condition. The results indicated that the quantitative retention (>90%) of the target ions at lower pH could be obtained on the adsorbent with a higher ratio of GO, indicating that GO played a key role in retention of the analytes in agreement with the pH-dependent properties of GO which was negatively charged over the pH range from 2 to 10.39,40 And the adsorption mechanism may be mainly due to the binding of metal ions with the negatively charged functional groups available on GO surfaces. It should be noted that GO–TiO2 composite with the ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 9
1 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were also synthesized and expected to be applied for the adsorption of target ions. But both of them caused high pressure in the SPE column. Considering the unique advantages of GO, the composite with a higher ratio of GO was preferred as the micro-column material. Hence, the GO–TiO2 (1
1 were also synthesized and expected to be applied for the adsorption of target ions. But both of them caused high pressure in the SPE column. Considering the unique advantages of GO, the composite with a higher ratio of GO was preferred as the micro-column material. Hence, the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite was adopted for all subsequent studies and the sample solution was adjusted to pH 5 for preconcentration of the target metal ions.
1) composite was adopted for all subsequent studies and the sample solution was adjusted to pH 5 for preconcentration of the target metal ions.
3.2 Characterization of the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1) composite
1) composite
FT-IR spectra of the GO, TiO2 and GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are shown in Fig. S1.† In the FT-IR spectra of the prepared GO, characteristic bands of 1065, 1229, 1384, 1627 and 1733 cm−1 were obviously observed, which were attributed to the C–O–C stretching peak, C–OH stretching vibrations, O–H deformation vibration of the C–OH group, the C
1) are shown in Fig. S1.† In the FT-IR spectra of the prepared GO, characteristic bands of 1065, 1229, 1384, 1627 and 1733 cm−1 were obviously observed, which were attributed to the C–O–C stretching peak, C–OH stretching vibrations, O–H deformation vibration of the C–OH group, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations and the C
C stretching vibrations and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode of the –COOH group, respectively. These features demonstrated the presence of the oxygen-containing groups on the self-prepared GO. For TiO2, a characteristic peak of 1621 cm−1 was observed, which was assigned to the stretching vibrations of –OH on the surface of TiO2, along with characteristic bands at about 1000 and 574 cm−1, ascribed to the Ti–O–Ti vibrations.41 In the FT-IR spectra of GO–TiO2 (1
O stretching mode of the –COOH group, respectively. These features demonstrated the presence of the oxygen-containing groups on the self-prepared GO. For TiO2, a characteristic peak of 1621 cm−1 was observed, which was assigned to the stretching vibrations of –OH on the surface of TiO2, along with characteristic bands at about 1000 and 574 cm−1, ascribed to the Ti–O–Ti vibrations.41 In the FT-IR spectra of GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), both the characteristic peaks of GO and TiO2 mentioned above appeared, and an absorption peak at 744 cm−1 was observed, which was ascribed to the combination of C–O–Ti vibration (at 798 cm−1)42 and Ti–O–Ti vibration. Besides, a wide peak at around 3409 cm−1, corresponding to the stretching vibrations of O–H, was observed in the spectra of GO, TiO2 and GO–TiO2 composite, probably indicating the presence of large quantity of moisture.
1), both the characteristic peaks of GO and TiO2 mentioned above appeared, and an absorption peak at 744 cm−1 was observed, which was ascribed to the combination of C–O–Ti vibration (at 798 cm−1)42 and Ti–O–Ti vibration. Besides, a wide peak at around 3409 cm−1, corresponding to the stretching vibrations of O–H, was observed in the spectra of GO, TiO2 and GO–TiO2 composite, probably indicating the presence of large quantity of moisture.
The composition of GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was further characterized with TGA/DTG in a nitrogen environment, as shown in Fig. S2.† For GO and GO–TiO2 (1
1) was further characterized with TGA/DTG in a nitrogen environment, as shown in Fig. S2.† For GO and GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite, the weight loss at low temperature (<100 °C) was assigned to the loss of the residual or adsorbed solvent. For GO, a sharp weight loss occurred around 196 °C, indicating the decomposition of oxygen groups, in agreement with the reported temperature.43 And for GO–TiO2 (1
1) composite, the weight loss at low temperature (<100 °C) was assigned to the loss of the residual or adsorbed solvent. For GO, a sharp weight loss occurred around 196 °C, indicating the decomposition of oxygen groups, in agreement with the reported temperature.43 And for GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite, an obvious weight loss occurred at 229 °C, which was mainly the loss of the functional groups on GO sheets. Compared with GO, the decomposition temperature of the composite increased about 30 °C, which may result from the interactions between TiO2 and GO.44 The TGA/DTG curves show that the weight losses of oxygen groups in GO and GO–TiO2 (1
1) composite, an obvious weight loss occurred at 229 °C, which was mainly the loss of the functional groups on GO sheets. Compared with GO, the decomposition temperature of the composite increased about 30 °C, which may result from the interactions between TiO2 and GO.44 The TGA/DTG curves show that the weight losses of oxygen groups in GO and GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite were about 35% and 17%, respectively, indicating that the weight percentage of GO in the composite was about 50%.
1) composite were about 35% and 17%, respectively, indicating that the weight percentage of GO in the composite was about 50%.
The XRD patterns of GO, TiO2 and GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are shown in Fig. S3.† The diffraction peak of GO appeared at around 2θ = 10.9° and interlayer spacing was about 0.81 nm. For the XRD of the self-prepared TiO2, the peaks at 2θ value of 25.62°, 37.98°, 48.14°, 54.12°/55.34°, 62.56°/62.96° and 68.8°/70.02° were indexed to (101), (004), (200), (105/211), (213/204) and (116/220) crystal planes of TiO2 in agreement with the anatase form (JCPDS no. 00-021-1272). In the XRD pattern of GO–TiO2 (1
1) are shown in Fig. S3.† The diffraction peak of GO appeared at around 2θ = 10.9° and interlayer spacing was about 0.81 nm. For the XRD of the self-prepared TiO2, the peaks at 2θ value of 25.62°, 37.98°, 48.14°, 54.12°/55.34°, 62.56°/62.96° and 68.8°/70.02° were indexed to (101), (004), (200), (105/211), (213/204) and (116/220) crystal planes of TiO2 in agreement with the anatase form (JCPDS no. 00-021-1272). In the XRD pattern of GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite, the diffraction peak of GO disappeared probably due to the disrupted layer-stacking regularity.45
1) composite, the diffraction peak of GO disappeared probably due to the disrupted layer-stacking regularity.45
The TEM images of GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite were obtained. As can be seen in Fig. 2(A), TiO2 was in situ grown on the GO layer with the size of 10–15 nm. High resolution TEM (Fig. 2(B)) and the selected area electron diffraction (SAED) results indicated that the prepared TiO2 was an anatase phase, consistent with the XRD pattern in Fig. S3(B).†
1) composite were obtained. As can be seen in Fig. 2(A), TiO2 was in situ grown on the GO layer with the size of 10–15 nm. High resolution TEM (Fig. 2(B)) and the selected area electron diffraction (SAED) results indicated that the prepared TiO2 was an anatase phase, consistent with the XRD pattern in Fig. S3(B).†
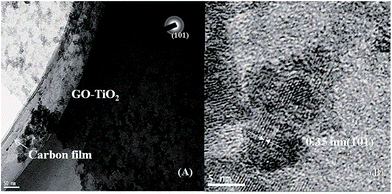 | ||
Fig. 2 (A) low magnification, and (B) high magnification TEM images of GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite. The inset of (A) shows a selected area electron diffraction (SAED) pattern. 1) composite. The inset of (A) shows a selected area electron diffraction (SAED) pattern. | ||
3.3 Optimization of the SPE procedure
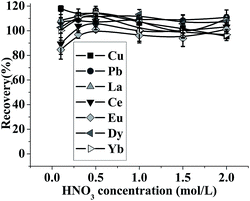 | ||
| Fig. 3 Effect of HNO3 concentration on the recovery of the analytes. pH: 5; the concentration of the metal ions: 0.5 μg mL−1; sample volume: 2.5 mL; eluent volume: 1.5 mL; flow rate: 0.5 mL min−1. | ||
The effect of eluent volume on the desorption of target ions was studied in the range of 0.3–1.0 mL by using 1 mol L−1 HNO3 as the eluent. The results are shown in Fig. S4.† As can be seen, quantitative recoveries (>90%) were obtained when the volume of HNO3 was above 0.7 mL. Therefore, 0.7 mL of eluent volume was selected for this study.
The influence of elution flow rate on recovery of the analytes was also investigated by keeping the eluent as 0.7 mL of 1 mol L−1 HNO3. The results in Fig. S5† indicate that the analytes could be recovered quantitatively (>90%) with the elution flow rate in the range of 0.35–1.5 mL min−1. And a flow rate of 1.2 mL min−1 was used for elution in further experiment.
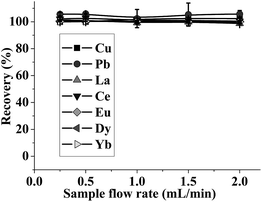 | ||
| Fig. 4 Effect of sample flow rate on recovery of the analytes. pH: 5; the concentration of the metal ions: 0.5 μg mL−1; sample volume: 2.5 mL. | ||
In order to obtain a high enrichment factor, a large volume of sample solution is required. The effect of sample volume on retention of the target ions was investigated by passing 7, 15, 30, 40, 60, 70 and 100 mL sample solutions containing 1.25 μg of Cu, Pb, La, Ce, Eu, Dy and Yb through the micro-column under the optimized conditions. The analytes on the column could be quantitatively desorbed when the sample volume was less than 70 mL, as shown in Fig. S6.† And a theoretical enrichment factor of 100 could be achieved. To trade off the enrichment factor and analytical speed, a sample volume of 7 mL and an elution volume of 0.7 mL were chosen in further experiment. Therefore, an enrichment factor of 10 and a sampling frequency of 12 h−1 were obtained in this work.
3.4 Co-existing ions interference
Various coexisting ions were added individually to 7 mL sample solutions containing 180 ng mL−1 Cu, Pb, La, Ce, Eu, Dy and Yb, respectively, and their effects on the recovery of analytes were investigated under the optimized conditions. The tolerance limit of the coexisting ions is defined as the largest amount making the recovery of the studied elements remain higher than 90%. The results summarized in Table 2 show that the developed method has a good tolerance to external interference and can be applied in the analysis of high-salinity samples.3.5 Regeneration of the prepared adsorbents
The regeneration of the prepared GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite was investigated by pumping 7 mL solution of the analytes through the GO–TiO2 (1
1) composite was investigated by pumping 7 mL solution of the analytes through the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) packed column, followed by passing through 0.7 mL of 1.0 mol L−1 HNO3 and 1 mL of 0.1 mol L−1 NH4Ac, repeatedly and continuously. It was observed that the packed column could be reused more than 90 runs without an obvious decrease in the recoveries of the analytes, demonstrating a good stability, acid-resistant ability and long lifetime for the prepared GO–TiO2 composite.
1) packed column, followed by passing through 0.7 mL of 1.0 mol L−1 HNO3 and 1 mL of 0.1 mol L−1 NH4Ac, repeatedly and continuously. It was observed that the packed column could be reused more than 90 runs without an obvious decrease in the recoveries of the analytes, demonstrating a good stability, acid-resistant ability and long lifetime for the prepared GO–TiO2 composite.
3.6 Dynamic adsorption & adsorption kinetics
For dynamic column operation, the breakthrough capacity represents the exhaustion point in terms of feed volume, after which the adsorbate leaks through into the effluent in gradually increasing amounts that could exceed the preset or desired leakage value.46,47 The breakthrough capacity of the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) adsorbent was investigated by sampling aqueous solution containing 10 μg mL−1 analyte at a flow rate of 2.0 mL min−1. It is assumed that breakthrough occurs at Ce/Ci = 0.01, where Ce is the metal concentration in the effluent and Ci is the corresponding initial concentration. As shown in Fig. S7,† the breakthrough capacities for Cu, Pb, La, Ce, Eu, Dy and Yb were calculated to be 0.8, 13.5, 3.8, 2.9, 2.8, 2.7 and 3.2 mg g−1, respectively.
1) adsorbent was investigated by sampling aqueous solution containing 10 μg mL−1 analyte at a flow rate of 2.0 mL min−1. It is assumed that breakthrough occurs at Ce/Ci = 0.01, where Ce is the metal concentration in the effluent and Ci is the corresponding initial concentration. As shown in Fig. S7,† the breakthrough capacities for Cu, Pb, La, Ce, Eu, Dy and Yb were calculated to be 0.8, 13.5, 3.8, 2.9, 2.8, 2.7 and 3.2 mg g−1, respectively.
The dynamic adsorption capacity, another important parameter for on-line application, was measured by passing a fixed concentration (10 μg mL−1) of the analyte solution through the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) packed micro-column at a constant flow rate of 2.0 mL min−1. The concentration of the analyte in the effluent was monitored by ICP-OES until the signal intensity of the effluent was stable. The adsorbed analyte on the GO–TiO2 (1
1) packed micro-column at a constant flow rate of 2.0 mL min−1. The concentration of the analyte in the effluent was monitored by ICP-OES until the signal intensity of the effluent was stable. The adsorbed analyte on the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite was then eluted with 1.0 mol L−1 HNO3. The dynamic adsorption capacities of the GO–TiO2 (1
1) composite was then eluted with 1.0 mol L−1 HNO3. The dynamic adsorption capacities of the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite for the target ions were listed in Table S1.† Along with some related SPE materials,11–13,15,18–21,37 the adsorption capacity of GO–TiO2 (1
1) composite for the target ions were listed in Table S1.† Along with some related SPE materials,11–13,15,18–21,37 the adsorption capacity of GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was at a comparatively high level among those reported adsorbents.
1) was at a comparatively high level among those reported adsorbents.
In order to further understand the adsorption kinetics of the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite, the experimental data were fitted with pseudo-first-order (1) and pseudo-second-order (2) models as expressed in the following equations, respectively.
1) composite, the experimental data were fitted with pseudo-first-order (1) and pseudo-second-order (2) models as expressed in the following equations, respectively.
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (1) |
| t/qt = 1/(k2qe2) + t/qe | (2) |
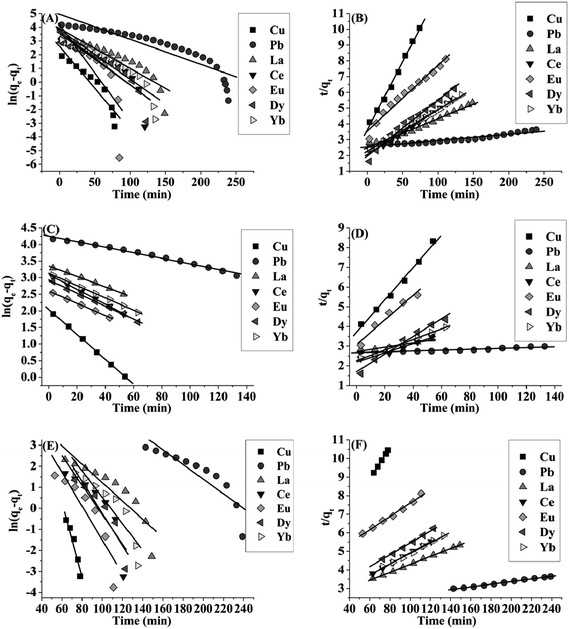
The dynamic adsorption behavior of each analyte (10 μg mL−1) on GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) adsorbent (50 mg) was studied using the above two models. Fig. 5 showed the linear plots of pseudo-first-order and pseudo-second-order adsorption for target ions. The correlation coefficient values (R2) of the two modes were listed in Table S2.† For all of the analytes, the overall adsorption process may involve two kinetic steps and was mainly controlled by the chemical reaction kinetics. The experimental data were fitted with the pseudo-first-order well in the former period (from 0 to about 50–60 min for Cu, La, Ce, Eu, Dy and Yb and 140 min for Pb) and the pseudo-second-order provided better correlation coefficient values in the latter period (from about 50–60 min for Cu, La, Ce, Eu, Dy and Yb and 140 min for Pb to adsorption equilibrium). In the initial period, there were sufficient binding sites on the GO–TiO2 (1
1) adsorbent (50 mg) was studied using the above two models. Fig. 5 showed the linear plots of pseudo-first-order and pseudo-second-order adsorption for target ions. The correlation coefficient values (R2) of the two modes were listed in Table S2.† For all of the analytes, the overall adsorption process may involve two kinetic steps and was mainly controlled by the chemical reaction kinetics. The experimental data were fitted with the pseudo-first-order well in the former period (from 0 to about 50–60 min for Cu, La, Ce, Eu, Dy and Yb and 140 min for Pb) and the pseudo-second-order provided better correlation coefficient values in the latter period (from about 50–60 min for Cu, La, Ce, Eu, Dy and Yb and 140 min for Pb to adsorption equilibrium). In the initial period, there were sufficient binding sites on the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite surface and so the kinetics may be mainly under diffusion control, following the pseudo-first-order model. As the functional groups on the GO–TiO2 (1
1) composite surface and so the kinetics may be mainly under diffusion control, following the pseudo-first-order model. As the functional groups on the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite surface being occupied, the adsorption kinetics was gradually controlled by chemical reaction, which can be well described with the pseudo-second-order model. For the whole process, the pseudo-second-order equation can provide better correlation coefficient and the rate-determining step was concluded to be chemical reaction involving the strong complexation of target ions with the oxygen-containing groups on the surface of GO–TiO2 (1
1) composite surface being occupied, the adsorption kinetics was gradually controlled by chemical reaction, which can be well described with the pseudo-second-order model. For the whole process, the pseudo-second-order equation can provide better correlation coefficient and the rate-determining step was concluded to be chemical reaction involving the strong complexation of target ions with the oxygen-containing groups on the surface of GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite.
1) composite.
3.7 Analytical performance of the FI on-line SPE-ICP-OES
The on-line analytical performance of the method was evaluated under the optimized conditions and the results were shown in Table 3. The linear ranges for target analytes were in the range of 0.5–1000 ng mL−1. The limits of detection (LODs) were determined as three times the standard deviation of the blank solution signal (n = 10). The LODs and the relative standard deviations (RSDs) of the method for Cu, Pb, La, Ce, Eu, Dy and Yb were 0.48, 2.64, 0.41, 0.24, 0.13, 0.26 and 0.21 ng mL−1 and 6.4, 9.8, 8.6, 3.2, 5.6, 4.5 and 6.2% (C = 10 ng mL−1, n = 7), respectively. As shown in Table S3,† the sample throughput and LODs of this method were satisfied and it was comparable with other on-line SPE-ICP-OES methods11–15,48 using TiO2 or carbon-based materials as adsorbents.| Analyte | Linear equation (ng mL−1) | R2 | Linear range (ng mL−1) | LODsa (n = 10) (ng mL−1) | RSD (%) (C = 10 ng mL−1, n = 7) |
|---|---|---|---|---|---|
| a LODs = 3σB/S (σB = standard deviation of 10 runs of the blank samples, S = line slope of calibration graph). | |||||
| Cu | Y = 0.120x + 0.1 | 0.995 | 1–1000 | 0.48 | 6.4 |
| Pb | Y = 0.027x − 0.1 | 0.996 | 10–1000 | 2.64 | 9.8 |
| La | Y = 0.077x + 0.1 | 0.990 | 1–1000 | 0.41 | 8.6 |
| Ce | Y = 0.070x + 0.3 | 0.993 | 1–1000 | 0.24 | 3.2 |
| Eu | Y = 0.568x − 0.1 | 0.996 | 0.5–1000 | 0.13 | 5.6 |
| Dy | Y = 0.182x + 1.3 | 0.994 | 1–1000 | 0.26 | 4.5 |
| Yb | Y = 0.498x − 0.2 | 0.991 | 1–1000 | 0.21 | 6.2 |
3.8 Sample analysis
The method was applied to the determination of target metals in natural water and sediment samples. The analytical data of the water and sediment samples along with recoveries of the spiked samples were shown in Table 4 and 5, respectively. The recoveries of target ions were 82.4–115.5% for water samples and 83.8–114.8% for sediment samples. It is noteworthy that the method can be applied in analysis of water with high salinity such as sea water.| Spiked (ng mL−1) | East Lake | Yangtze River | Sea water | ||||
|---|---|---|---|---|---|---|---|
| Found | Recovery (%) | Found | Recovery (%) | Found | Recovery (%) | ||
| a N.D.: not detected. | |||||||
| Cu | 0 | 1.7 ± 0.1 | — | 4.4 ± 0.2 | — | 7.8 ± 0.5 | — |
| 4 | 5.8 ± 0.5 | 102.3 | 8.9 ± 0.1 | 111.6 | 11.6 ± 0.3 | 95.1 | |
| 40 | 37.1 ± 1.6 | 88.5 | 37.4 ± 3.9 | 82.4 | 51.2 ± 1.4 | 108.4 | |
| 200 | 214.5 ± 5.7 | 106.4 | 227.6 ± 8.0 | 111.6 | 203.2 ± 5.5 | 97.7 | |
| Pb | 0 | N.D. | — | N.D. | — | N.D. | — |
| 20 | 19.6 ± 1.7 | 97.8 | 22.9 ± 1.4 | 114.7 | 17.6 ± 0.3 | 88.0 | |
| 200 | 219.3 ± 19.5 | 109.7 | 173.6 ± 18.8 | 86.8 | 214.8 ± 19.0 | 107.4 | |
| 1000 | 975.8 ± 89.3 | 97.6 | 1132.9 ± 73.5 | 113.3 | 987.2 ± 68.3 | 98.7 | |
| La | 0 | N.D. | — | N.D. | — | N.D. | — |
| 4 | 3.6 ± 0.1 | 90.0 | 3.7 ± 0.3 | 92.6 | 3.6 ± 0.2 | 90.4 | |
| 40 | 39.4 ± 1.8 | 98.6 | 37.7 ± 2.9 | 94.2 | 43.5 ± 1.3 | 108.6 | |
| 200 | 191.2 ± 4.9 | 95.6 | 200.4 ± 9.0 | 100.2 | 222.4 ± 5.0 | 111.2 | |
| Ce | 0 | N.D. | — | N.D. | — | N.D. | — |
| 4 | 4.2 ± 0.2 | 105.1 | 4.9 ± 0.1 | 102.1 | 3.6 ± 0.2 | 90.4 | |
| 40 | 45.9 ± 1.6 | 114.7 | 45.4 ± 4.3 | 111.3 | 42.2 ± 3.1 | 105.5 | |
| 200 | 206.9 ± 3.9 | 103.4 | 231.0 ± 8.3 | 115.1 | 191.2 ± 4.7 | 95.6 | |
| Eu | 0 | N.D. | — | N.D. | — | N.D. | — |
| 4 | 4.0 ± 0.2 | 92.2 | 3.8 ± 0.06 | 95.8 | 3.7 ± 0.2 | 92.8 | |
| 40 | 40.9 ± 0.9 | 101.5 | 34.7 ± 2.3 | 86.8 | 45.2 ± 1.6 | 113.0 | |
| 200 | 224.5 ± 16.2 | 112.1 | 200.5 ± 5.9 | 100.2 | 176.7 ± 7.2 | 88.4 | |
| Dy | 0 | N.D. | — | N.D. | — | N.D. | — |
| 4 | 4.6 ± 0.05 | 115.5 | 4.1 ± 0.2 | 102.5 | 3.4 ± 0.1 | 85.8 | |
| 40 | 41.1 ± 4.4 | 102.7 | 36.4 ± 1.0 | 91.0 | 36.2 ± 0.5 | 90.5 | |
| 200 | 181.3 ± 9.3 | 90.6 | 208.2 ± 17.1 | 104.1 | 176.5 ± 8.1 | 88.3 | |
| Yb | 0 | N.D. | — | N.D. | — | N.D. | — |
| 4 | 3.5 ± 0.2 | 87.1 | 3.4 ± 0.2 | 85.3 | 4.1 ± 0.1 | 102.2 | |
| 40 | 38.3 ± 2.1 | 95.8 | 34.4 ± 3.8 | 86.1 | 41.3 ± 1.0 | 103.2 | |
| 200 | 219.5 ± 12.2 | 109.8 | 198.8 ± 1.4 | 99.4 | 224.0 ± 19.6 | 112.0 | |
| Analyte | Spiked (μg g−1) | East Lake sediment | Yangtze River sediment | ||
|---|---|---|---|---|---|
| Found | Recovery (%) | Found | Recovery (%) | ||
| a N.D.: not detected. | |||||
| Cu | 0 | 39.2 ± 3.5 | — | 18.3 ± 1.2 | — |
| 80 | 106.2 ± 6.4 | 83.8 | 97.0 ± 7.9 | 98.3 | |
| Pb | 0 | 62.2 ± 6.1 | — | 45.5 ± 3.2 | — |
| 400 | 487.1 ± 33.7 | 106.2 | 434.8 ± 21.4 | 97.3 | |
| La | 0 | 32.1 ± 3.2 | — | 12.6 ± 0.2 | — |
| 80 | 122.6 ± 8.0 | 113.1 | 93.7 ± 8.8 | 101.4 | |
| Ce | 0 | 40.0 ± 2.0 | — | 57.2 ± 3.4 | — |
| 80 | 131.9 ± 8.1 | 114.8 | 138.1 ± 0.3 | 101.0 | |
| Eu | 0 | 1.83 ± 0.1 | — | N.D. | — |
| 80 | 91.6 ± 6.3 | 112.2 | 83.5 ± 5.4 | 104.4 | |
| Dy | 0 | 2.65 ± 0.2 | — | 1.8 ± 0.1 | — |
| 80 | 78.5 ± 6.6 | 94.8 | 70.2 ± 4.5 | 85.5 | |
| Yb | 0 | 1.2 ± 0.1 | — | N.D. | — |
| 80 | 80.0 ± 6.1 | 98.5 | 75.4 ± 4.9 | 94.2 | |
In order to validate the accuracy of the proposed method, the certified reference material GBW07301a (Stream sediment) was analyzed. The results were given in Table 6. As can be seen, the determined values obtained by the proposed method were in good agreement with the certified values.
4 Conclusion
A novel adsorbent of GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite was prepared and used as a micro-column packing material for on-line separation/preconcentration of heavy metals and REEs in environmental water and sediment samples. The adsorption behavior of target ions on GO–TiO2 (1
1) composite was prepared and used as a micro-column packing material for on-line separation/preconcentration of heavy metals and REEs in environmental water and sediment samples. The adsorption behavior of target ions on GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite has been studied systematically, and it was found that the GO–TiO2 (1
1) composite has been studied systematically, and it was found that the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite showed a high adsorption capacity for the metal ions, and the overall adsorption process may involve two kinetic steps and chemical reaction played a dominant role. As the GO–TiO2 (1
1) composite showed a high adsorption capacity for the metal ions, and the overall adsorption process may involve two kinetic steps and chemical reaction played a dominant role. As the GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite exhibited good stability and strong anti-interference capability, the established method has been successfully applied in the analysis of metal ions in high-salinity water (sea water) and sediment samples. The GO–TiO2 (1
1) composite exhibited good stability and strong anti-interference capability, the established method has been successfully applied in the analysis of metal ions in high-salinity water (sea water) and sediment samples. The GO–TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite has high chemical and thermal stability and is expected to be an ideal material in the evaluation of environmental pollution.
1) composite has high chemical and thermal stability and is expected to be an ideal material in the evaluation of environmental pollution.
Acknowledgements
The authors would like to thank the National Science Foundation of China (no. 21075095) and Science Fund for Creative Research Groups of NSFC (nos 20621502, 20921062) for their financial supports.References
- M. Hultberg, A. Isaksson, A. Andersson and B. Hultberg, Chem.-Biol. Interact., 2007, 167, 56–62 CrossRef CAS PubMed.
- H. W. Gao, T. Zeng, L. T. Pan, L. Chen, M. L. Li and Y. Yuan, Microchim. Acta, 2007, 158, 335–344 CrossRef CAS.
- X. L. Shen, K. Lee and R. Konig, Toxicology, 2001, 169, 67–80 CrossRef CAS.
- N. M. Mubarak, J. N. Sahu, E. C. Abdullah and N. S. Jayakumar, Sep. Purif. Rev., 2014, 43, 311–338 CrossRef CAS.
- H. W. Gao, F. F. Chen, L. Chen, T. Zeng, L. T. Pan, J. H. Li and H. F. Luo, Anal. Chim. Acta, 2007, 587, 52–59 CrossRef CAS PubMed.
- X. T. Zhao, T. Zeng, X. Y. Li, Z. J. Hu, H. W. Gao and Z. Xie, Carbohydr. Polym., 2012, 89, 185–192 CrossRef CAS PubMed.
- M. D. A. Korn, J. B. de Andrade, D. S. de Jesus, V. A. Lemos, M. Bandeira, W. N. L. dos Santos, M. A. Bezerra, F. A. C. Amorim, A. S. Souza and S. L. C. Ferreira, Talanta, 2006, 69, 16–24 CrossRef CAS PubMed.
- S. Zaichick, V. Zaichick, V. Karandashev and S. Nosenko, Metallomics, 2011, 3, 186–194 RSC.
- S. Porru, D. Placidi, C. Quarta, E. Sabbioni, R. Pietra and S. Fortaner, J. Trace Elem. Med. Biol., 2001, 14, 232–236 CAS.
- H. Zhang, J. Feng, W. F. Zhu, C. Q. Liu, S. Q. Xu, P. P. Shao, D. S. Wu, W. J. Yang and J. H. Gu, Biol. Trace Elem. Res., 2000, 73, 1–17 CrossRef CAS.
- D. H. Chen, B. Hu, M. He and C. Z. Huang, Microchem. J., 2010, 95, 90–95 CrossRef CAS PubMed.
- C. Z. Huang, Z. C. Jiang and B. Hu, Talanta, 2007, 73, 274–281 CrossRef CAS PubMed.
- P. Liang, B. Hu, Z. C. Jiang, Y. C. Qin and T. Y. Peng, J. Anal. At. Spectrom., 2001, 16, 863–866 RSC.
- P. Liang, Y. C. Qin, B. Hu, T. Y. Peng and Z. C. Jiang, Anal. Chim. Acta, 2001, 440, 207–213 CrossRef CAS.
- L. H. Yang, B. Hu, Z. C. Jiang and H. L. Pan, Microchim. Acta, 2004, 144, 227–231 CrossRef CAS.
- M. M. Silva, M. A. Z. Arruda, F. J. Krug, P. V. Oliveira, Z. F. Queiroz, M. Gallego and M. Valcarcel, Anal. Chim. Acta, 1998, 368, 255–263 CrossRef CAS.
- S. Z. Chen, M. F. Xiao, D. B. Lu and X. L. Zhan, Anal. Lett., 2007, 40, 2105–2115 CrossRef CAS.
- S. Z. Chen, C. Liu, M. Yang, D. B. Lu, L. Zhu and Z. Wang, J. Hazard. Mater., 2009, 170, 247–251 CrossRef CAS PubMed.
- P. Liang, Y. Liu and L. Guo, Spectrochim. Acta, Part B, 2005, 60, 125–129 CrossRef PubMed.
- E. Sahmetlioglu, E. Yilmaz, E. Aktas and M. Soylak, Talanta, 2014, 119, 447–451 CrossRef CAS PubMed.
- Y. K. Wang, S. T. Gao, X. H. Zang, J. C. Li and J. J. Ma, Anal. Chim. Acta, 2012, 716, 112–118 CrossRef CAS PubMed.
- E. Yavuz, S. Tokalioglu, H. Sahan and S. Patat, RSC Adv., 2013, 3, 24650–24657 RSC.
- M. D. Stoller, S. J. Park, Y. W. Zhu, J. H. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- G. X. Zhao, X. M. Ren, X. Gao, X. L. Tan, J. X. Li, C. L. Chen, Y. Y. Huang and X. K. Wang, Dalton Trans., 2011, 40, 10945–10952 RSC.
- S. T. Yang, Y. L. Chang, H. F. Wang, G. B. Liu, S. Chen, Y. W. Wang, Y. F. Liu and A. N. Cao, J. Colloid Interface Sci., 2010, 351, 122–127 CrossRef CAS PubMed.
- R. Sitko, E. Turek, B. Zawisza, E. Malicka, E. Talik, J. Heimann, A. Gagor, B. Feist and R. Wrzalik, Dalton Trans., 2013, 42, 5682–5689 RSC.
- Y. B. Sun, Q. Wang, C. L. Chen, X. L. Tan and X. K. Wang, Environ. Sci. Technol., 2012, 46, 6020–6027 CrossRef CAS PubMed.
- D. Y. Deng, X. M. Jiang, L. Yang, X. D. Hou and C. B. Zheng, Anal. Chem., 2014, 86, 758–765 CrossRef CAS PubMed.
- S. W. Su, B. B. Chen, M. He, B. Hu and Z. W. Xiao, Talanta, 2014, 119, 458–466 CrossRef CAS PubMed.
- T. Yang, L. H. Liu, J. W. Liu, M. L. Chen and J. H. Wang, J. Mater. Chem., 2012, 22, 21909–21916 RSC.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771–778 CrossRef CAS.
- R. Sitko, B. Zawisza, E. Talik, P. Janik, G. Osoba, B. Feist and E. Malick, Anal. Chim. Acta, 2014, 834, 22–29 CrossRef CAS PubMed.
- Q. Liu, J. B. Shi, J. T. Sun, T. Wang, L. X. Zeng and G. B. Jiang, Angew. Chem., Int. Ed., 2011, 50, 5913–5917 CrossRef CAS PubMed.
- K. Tani and Y. Suzuki, J. Chromatogr. A, 1996, 722, 129–134 CrossRef CAS.
- E. Vassileva, I. Proinova and K. Hadjiivanov, Analyst, 1996, 121, 607–612 RSC.
- S. Z. Chen, S. P. Zhu and D. B. Lu, Microchem. J., 2013, 110, 89–93 CrossRef CAS PubMed.
- Y. Y. Liang, H. L. Wang, H. S. Casalongue, Z. Chen and H. J. Dai, Nano Res., 2010, 3, 701–705 CrossRef CAS PubMed.
- C. J. Shih, S. C. Lin, R. Sharma, M. S. Strano and D. Blankschtein, Langmuir, 2012, 28, 235–241 CrossRef CAS PubMed.
- I. Chowdhury, M. C. Duch, N. D. Mansukhani, M. C. Hersam and D. Bouchard, Environ. Sci. Technol., 2013, 47, 6288–6296 CAS.
- C. Y. Hou, Q. H. Zhang, Y. G. Li and H. Z. Wang, J. Hazard. Mater., 2012, 205, 229–235 CrossRef PubMed.
- S. Sakthivel and H. Kisch, Angew. Chem., Int. Ed., 2003, 42, 4908–4911 CrossRef CAS PubMed.
- M. J. McAllister, J. L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, M. Herrera-Alonso, D. L. Milius, R. Car, R. K. Prud'homme and I. A. Aksay, Chem. Mater., 2007, 19, 4396–4404 CrossRef CAS.
- Y. C. Lee and J. W. Yang, J. Ind. Eng. Chem., 2012, 18, 1178–1185 CrossRef CAS PubMed.
- J. Liu, H. Bai, Y. Wang, Z. Liu, X. Zhang and D. D. Sun, Adv. Funct. Mater., 2010, 20, 4175–4181 CrossRef CAS.
- X. Wang and R. M. Barnes, J. Anal. At. Spectrom., 1989, 4, 509–518 RSC.
- Y. Liu, Y. Li and X. P. Yan, Adv. Funct. Mater., 2008, 18, 1536–1543 CrossRef CAS.
- E. A. Takara, S. D. Pasini-Cabello, S. Cerutti, J. A. Gasquez and L. D. Martinez, J. Pharm. Biomed. Anal., 2005, 39, 735–739 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13333a |
| This journal is © The Royal Society of Chemistry 2015 |