Temperature: a nonnegligible factor for the formation of a structurally stable, self-assembled reduced graphite oxide hydrogel
Yang Liu,
Cheng-Lu Liang,
Rui-Ying Bao,
Guo-Qiang Qi,
Wei Yang*,
Bang-Hu Xie and
Ming-Bo Yang
College of Polymer Science and Engineering, Sichuan University, State Key Laboratory of Polymer Materials Engineering, Chengdu, 610065, Sichuan, China. E-mail: weiyang@scu.edu.cn; Fax: +86 28 8546 0130; Tel: +86 28 8546 0130
First published on 19th November 2014
Abstract
The three-dimensional (3D) architecture of reduced graphite oxide (rGO) hydrogels is of interest in applications such as supercapacitors, soft machines and regenerative medicine, etc. The structural stability of the rGO hydrogel is the foundation for these applications. However, little attention has been paid to this issue. Here, the structural and performance stabilities of rGO hydrogels prepared at different temperatures were investigated in detail. It was found that 40 °C was the most effective condition for the reduction of graphite oxide, as the reducibility of vitamin C was embodied successfully and the network of the rGO hydrogel was formed. The rGO hydrogel prepared at 40 °C showed the best structural stability with time, the lowest electrical resistance and the highest mechanical strength. These results provide guidance for the synthesis of structurally stable rGO hydrogels and their further applications in electrical devices.
1. Introduction
Two-dimensional (2D) graphene and its derivatives have been regarded as one of the most promising materials for their prominent mechanical,1,2 thermal,3 electrical properties4–6 and good biocompatibility.7,8 With respect to 2D monolayer graphene, graphite-based hydrogels, consisting of a 3D cross-linked network with high water content, exhibit numerous advantages such as high porosity, good thermal stability, and special electronic and mechanical properties.9–12 A number of potential applications of these hydrogels have been found in various technological fields such as tissue engineering,13 soft machines,14,15 gel explosives,16 regenerative medicine,17 supercapacitors,18,19 and drug delivery.20,21Good structural stability of graphite or graphite oxide based hydrogels is the cornerstone for their successful applications.22 Many scholars have mixed graphite and graphite oxide (GO) with polymers or other nanoparticles to obtain stable hydrogels. These polymers or nanoparticles can not only functionalize the composite materials but also stabilize the hydrogels.23 For instance, Adhikari and coworkers obtained reduced graphite oxide based functional hybrids containing metal nanoparticle and the hybrid was used as catalyst for the reduction of aromatic nitro to amino group.24 Bai et al. studied GO/poly(vinyl alcohol) composite hydrogels which were utilized for selective releasing of drug at physiological PH.25 Zu and coworkers employed the pluronic copolymer as the solubilizing agent to stabilize the chemically prepared graphite based hydrogels. Nonetheless, the electrical conductivity of the hydrogels decreased with the added copolymer.26
Some scholars also sought to stabilize graphite based hydrogels by optimizing synthetic methods. Xu prepared a mechanically strong and thermally stable self-assembled reduced graphite oxide (rGO) hydrogel via a convenient one-step hydrothermal method.9 However, the strict reaction condition is not appropriate for mass production. As we know, the chemical redox process is the most convenient way to obtain rGO hydrogel. During chemical redox process, the structure of GO, the formation temperature and reaction time all play an important role in the formation of hydrogels.27 The influence of GO structure28 and reaction time29 during this process have been studied in detail. In synthetic copolymer, a sol–gel transition with spontaneous physical gelation upon heating12,30–32 has been widely reported. However, as far as we know, little attention had been paid to the effect of the temperature on the structural stability with time of rGO hydrogel during the formation process. Here, we studied the effect of the formation temperature on the structural stability of rGO hydrogels via a chemical reduction of GO in aqueous solution under atmospheric pressure. The mechanical and electrical properties of the rGO hydrogels were investigated to reveal the structural stability.
2. Experimental section
2.1 Materials
Natural graphite flakes with an average particle size of 200 meshes and a purity of over 99.9% were purchased from Shenghua Research Institute (Changsha, China). Distilled water (H2O), concentrate sulfuric acid (H2SO4), potassium permanganate (KMnO4), hydrogen peroxide (H2O2), potassium persulfate (K2S2O8) and phosphorus pentoxide (P2O5) supplied by Haihong Chemical Reagents Company (Chengdu, China) were used as received. All the reagents are of analytical grade. Graphite was dried at 60 °C in a vacuum oven for 24 h before use.2.2 Synthesis of rGO hydrogel
GO was synthesized from natural graphite powder using a modified Hummers method in which a two-step oxidation was employed.33 Then the GO aqueous suspension was regulated to 5 mg ml−1. 100 ml GO suspension was added to a 250 ml beaker and sonicated for 30 min at 25 °C under continuous stirring. After that, 2.5 g sodium ascorbate powder was added into the beaker slowly and the mixture was kept sonicating for another 5 min. The solution was then kept at 30, 40 and 50 °C for 24 h respectively to complete the self-assembly process of the rGO hydrogels. It should be pointed out that the reaction time is an important factor influencing the formation of stable hydrogels. But according to Sheng's report,27 the reaction time we adopted is more than enough for the formation of hydrogel. The mechanical and electrical properties of rGO hydrogels were characterized by dynamic rheological tests and accompanying resistance measurements. The structures of GO and rGO were characterized with their corresponding freeze-dried samples. The rGO hydrogels prepared at 30, 40 and 50 °C were coded as rGO-30, rGO-40 and rGO-50 respectively.2.3 Characterization of rGO hydrogels
Dynamic rheological tests were carried out using a rotational rheometer (AR2000EX, TA instruments, USA) with parallel plates (25 mm diameter) at 25 °C, and a resistance measurement device, Keithley 6517B, was attached to the rotational rheometer. The resistance (R) was measured at a dc voltage of 0.1 V during rheology test.Freeze-dried GO and rGO aerogels which kept their primary structures in the hydrogels were characterized by the following methods. The morphologies of the samples were characterized using field-emission scanning electron microscopy (SEM, JSM-5900LV, Japan) and transmission electron microscopy (TEM, Tecnai G2 F20 S-TWIN, FEI, USA). X-ray diffraction (XRD) was carried out at room temperature with a DX-1000 Scintag diffractometer. The aerogels were scanned over the range of diffraction angle 2θ = 2–45° at a scan speed of 3 °C min−1 using CuKα radiation (λ = 0.154 nm) with a filament voltage of 45 kV and current of 40 mA. XPS (X-ray photoelectron spectroscopy) measurements were performed on an XSAM800 (Kratos Company, UK) instrument with AlKα radiation (hν = 1486.6 eV) and XPS peak 41 software (Chemistry, CUHK) was used to calculate the atomic concentrations. Fourier-transform infrared (FTIR) spectroscopy was performed over the wave number range of 4000–400 cm−1 using a Nicolet 6700 FTIR spectrometer (Nicolet Instrument Company, USA).
3. Results and discussion
3.1 The mechanical and electrical properties of rGO hydrogel
The mechanical properties and structural stability with time of rGO hydrogels was studied by dynamic rheology. In Fig. 1a, the dynamic storage modulus (G′) of rGO-40 and rGO-50 hydrogel not only show little change with increased frequency but also are higher than the loss storage modulus (G′′) of that in Fig. 1b, indicating the formation of network of hydrogels at 40 °C and 50 °C.34 Furthermore, the G′ value of rGO-40 hydrogel is almost 50× higher than that of rGO-50 hydrogel, demonstrating that the 3D porous network of rGO-40 hydrogel is more integral. The G′ and G′′ value of rGO-40 hydrogel also show no change as shown in Fig. 1c and d over time, which also confirms the self-assembled rGO hydrogel architecture is structurally stable with time.A stable self-assembled rGO network of the hydrogels will surely lead to a stable electric conductive pathway, so the electrical resistances of the hydrogels were also measured during time sweep and the results are displayed in Fig. 2. As time goes on, the electrical resistance of rGO-30 and rGO-50 decreases remarkably, which can be ascribed to directional arrangement of graphene nanosheets along the direction of current owing to the unstable network in the hydrogels. In contrast, the electrical resistance of rGO-40 hydrogel shows little changes over time, which clearly confirms that the conductive network is stable in the presence of an electrical field. Apparently, the electrical resistance values of the three hydrogels are very low compared with that of their solvent, distilled water. Especially for rGO-40 hydrogel, the volume electrical resistance is as low as 700 Ω cm, which provides the potential to be used in various electrical devices.11,35
 | ||
| Fig. 2 The electrical resistance vs. time of H2O and the three rGO hydrogels synthesized at different temperature. | ||
3.2 The network structures and formation mechanism of rGO hydrogel
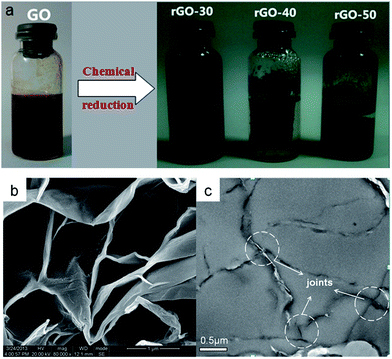
Fig. 3a shows the photographs of an aqueous mixture of GO and the three rGO hydrogels prepared at different temperatures. It is clear that rGO-40 hydrogel is the strongest and presents as a free-standing cylinder. The microstructures of as-prepared rGO hydrogels were characterized by SEM and TEM. As shown in Fig. 3b, a self-assembled, well-jointed network structure of rGO-40 hydrogel can be seen clearly. The TEM sample was ultramicrotomed perpendicular to the graphite sheets, so we can see the joints between layers in Fig. 3c. The 3D architecture may be formed as Scheme 1, and the self-assembly process was driven by the hydrophobic and π–π interaction between layers.36,37 At the beginning of the formation of the hydrogel, some graphene oxide sheets suspended in water by the hydrophilic interaction.38 When the reducing agent Vc (vitamin C) was added to the solution, the reduction of GO occurred. An elevated temperature is helpful for the coalescence and self-assembly of reduced graphene oxide sheets, which leads to more integral network and the higher electrical conductivity of rGO-40 and rGO-50 hydrogel than rGO-30 hydrogel. However, the reducibility of Vc would decrease at high temperature, as Vc is prone to decompose at temperatures higher than 60 °C as reported.39 The decreased reducibility of Vc leaded to the incomplete reduction of GO at 50 °C, as evidenced by the XPS and FT-IR results shown later. During the reduction of GO, a large amount of water was expelled from the porous structure, but much residual water was included in the gel and compact 3D architectures could be formed not only by π–π stacking interaction but also by remnant hydrophilicity. | ||
| Scheme 1 The mechanism of self-assembled 3D network for rGO hydrogel during the reduction of GO by vitamin C in aqueous solution. | ||
3.3 The characteristic of the chemical structure of GO and rGO hydrogel
The compact 3D architectures of the hydrogels were constructed by π-conjugated system recovered from GO sheets upon reduction, as confirmed by the XRD patterns shown in Fig. 4. The interlayer spacing of the three rGO hydrogels was calculated to be 0.37 nm. This value is much lower than that of GO precursor (0.93 nm) and natural graphite (0.33 nm). These results suggest the existence of residual oxygenated functional groups on reduced GO sheets and the presence of π–π stacking between graphene sheets in the rGO.40,41 Because of the interactions between these residual hydrophilic oxygen-containing functional groups and hydrogen oxide by hydrogen bonding, the rGO hydrogels networks could encapsulate water during the process of self-assembly. The broad XRD peak of rGO hydrogels indicates the framework is composed of few-layer stacked graphene sheets and reflects the poor ordering of graphene sheets. For GO, a sharp diffraction peak appeared at 9.5° corresponding to a d-spacing of 0.93 nm. However, after chemical reduction, this peak disappeared and a wide diffraction peak appeared at 24° with an interlayer spacing of 0.37 nm. Apparently, efficient exfoliation of the multilayers occurred during chemical redox of GO.Reduction of GO by Vc was confirmed by XPS and FT-IR, together with a color change of the reaction mixture from brown to black as shown in Fig. 1. C1s XPS spectra of these samples were presented in Fig. 5. For GO, four different peaks centered at 284.6, 286.5, 287.9 and 289.2 eV were observed, corresponding to C–C and/or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in aromatic rings, C–OH (epoxy and alkoxy), C
C in aromatic rings, C–OH (epoxy and alkoxy), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups respectively.33,36 After chemical reduction, the intensity of the oxygen-containing groups decreased significantly. Compared with rGO-30 and rGO-50, the peak of C–O and O–C
O groups respectively.33,36 After chemical reduction, the intensity of the oxygen-containing groups decreased significantly. Compared with rGO-30 and rGO-50, the peak of C–O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O reduced more obviously and the oxygenated group of C
O reduced more obviously and the oxygenated group of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O almost disappeared, which indicates rGO-40 was the most efficiently reduced.42 The value of C/O ratio in Table 1 demonstrated the results as well. The most successful reduction of rGO-40 may result from that Vc performs a best reducibility and GO sheets can move more actively to overlap and coalesce during the reduction at 40 °C.
O almost disappeared, which indicates rGO-40 was the most efficiently reduced.42 The value of C/O ratio in Table 1 demonstrated the results as well. The most successful reduction of rGO-40 may result from that Vc performs a best reducibility and GO sheets can move more actively to overlap and coalesce during the reduction at 40 °C.
 | ||
| Fig. 5 The C1s XPS spectra of GO (a) and products obtained from reduction of GO with Vc at different temperature: 40 °C (b), 30 °C (c), 50 °C (d). | ||
| C (%) | O (%) | C/O | |
|---|---|---|---|
| GO | 70.85 | 29.15 | 2.4 |
| rGO-30 | 74.29 | 25.71 | 2.9 |
| rGO-40 | 80.01 | 19.99 | 4.2 |
| rGO-50 | 79.31 | 20.69 | 3.8 |
The removal of the oxygen-containing functional groups located on the basal plane of GO can be reflected from the change of diffraction peak in FT-IR as well. As shown in Fig. 6, after reduction of GO with Vc, the intensities of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations peak at 1724 cm−1 and O–H in-plane bending vibrations from hydroxyl groups at 1410 cm−1 decreased remarkably. Furthermore, the C–O (epoxy) stretching vibration peak at 1226 cm−1 and C–O stretching vibration associated to alkoxy peak at 1025 cm−1 disappeared and a new wide diffraction peak at 1620 cm−1 corresponding to C
O stretching vibrations peak at 1724 cm−1 and O–H in-plane bending vibrations from hydroxyl groups at 1410 cm−1 decreased remarkably. Furthermore, the C–O (epoxy) stretching vibration peak at 1226 cm−1 and C–O stretching vibration associated to alkoxy peak at 1025 cm−1 disappeared and a new wide diffraction peak at 1620 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration from unoxidized graphitic domains appeared. These observations confirmed that a large proportion of oxygen functionalities in GO were removed. Especially in rGO-40, the O–C
C stretching vibration from unoxidized graphitic domains appeared. These observations confirmed that a large proportion of oxygen functionalities in GO were removed. Especially in rGO-40, the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O almost disappeared which demonstrated Vc showed the highest reducibility at 40 °C. Not only C
O almost disappeared which demonstrated Vc showed the highest reducibility at 40 °C. Not only C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond could be restored but also all oxygenated functional groups of GO could be reduced, and then the reduced graphite oxide sheets could self-assemble into a compact network architecture.
C bond could be restored but also all oxygenated functional groups of GO could be reduced, and then the reduced graphite oxide sheets could self-assemble into a compact network architecture.
The structure characteristics of GO and rGO hydrogels demonstrated that rGO hydrogel prepared at 40 °C was reduced most successfully. There are some reasons below. To begin with, GO sheets at 40 °C can move more actively to overlap and coalesce during the reduction than at 30 °C. In addition, the reducibility of Vc at 40 °C is higher than that at 50 °C in GO aqueous solution, which leads to better reduction of GO. So rGO-40 hydrogel shows the highest electrical conductivity and mechanical strength and the best structural stability among three rGO hydrogels.
4. Conclusion
In conclusion, we have demonstrated that temperature is an important factor for the network formation of rGO hydrogel. Compared with hydrogels synthesized at 30 °C and 50 °C, rGO hydrogel prepared at 40 °C performed the highest mechanical strength and the lowest electrical resistance and best structural stability with time. Since GO was reduced most successful by Vc at 40 °C which was shown clearly in the results of structural characteristics by XPS and FT-IR. This study will provide some guidance on the choosing of formation temperature of structurally stable rGO hydrogels and further applications in electrical devices.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (Grant nos 51422305 and 51121001), the MOST (Grant no. 2012CB025902) and the Innovation Team Program of Science & Technology Department of Sichuan Province (Grant 2013TD0013). Mr Chao-liang Zhang, working at the State Key Laboratory of Oral Medicine of China, was also acknowledged for his kind help in FE-SEM observations.Notes and references
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed.
- S. Park, K.-S. Lee, G. Bozoklu, W. Cai, S. T. Nguyen and R. S. Ruoff, ACS Nano, 2008, 2, 572–578 CrossRef CAS PubMed.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS PubMed.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270–274 CrossRef CAS PubMed.
- C. X. Guo, H. B. Yang, Z. M. Sheng, Z. S. Lu, Q. L. Song and C. M. Li, Angew. Chem., 2010, 49, 3014–3017 CrossRef CAS PubMed.
- G. M. Rutter, J. N. Crain, N. P. Guisinger, T. Li, P. N. First and J. A. Stroscio, Science, 2007, 317, 219–222 CrossRef CAS PubMed.
- V. Singh, D. Joung, L. Zhai, S. Das, S. I. Khondaker and S. Seal, Prog. Mater. Sci., 2011, 56, 1178–1271 CrossRef CAS PubMed.
- H. Luo, G. Xiong, Z. Yang, S. R. Raman, H. Si and Y. Wan, RSC Adv., 2014, 4, 14369–14372 RSC.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS PubMed.
- A. Guiseppi-Elie, Biomaterials, 2010, 31, 2701–2716 CrossRef CAS PubMed.
- P. Calvert, Adv. Mater., 2009, 21, 743–756 CrossRef CAS.
- L. Yu and J. Ding, Chem. Soc. Rev., 2008, 37, 1473–1481 RSC.
- S. Sayyar, E. Murray, B. C. Thompson, S. Gambhir, D. L. Officer and G. G. Wallace, Carbon, 2013, 52, 296–304 CrossRef CAS PubMed.
- J. P. Gong, Soft Matter, 2010, 6, 2583–2590 RSC.
- P. Huang, W. Chen and L. Yan, Nanoscale, 2013, 5, 6034–6039 RSC.
- C. X. Guo, Z. S. Lu, Y. Lei and C. M. Li, Electrochem. Commun., 2010, 12, 1237–1240 CrossRef CAS PubMed.
- B. V. Slaughter, S. S. Khurshid, O. Z. Fisher, A. Khademhosseini and N. A. Peppas, Adv. Mater., 2009, 21, 3307–3329 CrossRef CAS PubMed.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- C. Du, Z. Yao, Y. Chen, H. Bai and L. Li, RSC Adv., 2014, 4, 9133–9138 RSC.
- D. Ma, J. Lin, Y. Chen, W. Xue and L.-M. Zhang, Carbon, 2012, 50, 3001–3007 CrossRef CAS PubMed.
- B. Adhikari and A. Banerjee, Soft Matter, 2011, 7, 9259–9266 RSC.
- C. K. Kuo and P. X. Ma, Biomaterials, 2001, 22, 511–521 CrossRef CAS.
- Z. Li, J. Shen, H. Ma, X. Lu, M. Shi, N. Li and M. Ye, Soft Matter, 2012, 8, 3139–3145 RSC.
- B. Adhikari, A. Biswas and A. Banerjee, ACS Appl. Mater. Interfaces, 2012, 4, 5472–5482 CAS.
- H. Bai, C. Li, X. Wang and G. Shi, Chem. Commun., 2010, 46, 2376–2378 RSC.
- S.-Z. Zu and B.-H. Han, J. Phys. Chem. C, 2009, 113, 13651–13657 CAS.
- K.-X. Sheng, Y.-X. Xu, C. Li and G.-Q. Shi, New Carbon Mater., 2011, 26, 9–15 CrossRef CAS.
- Y. Liu, G.-Q. Qi, C.-L. Liang, R.-Y. Bao, W. Yang, B.-H. Xie and M.-B. Yang, J. Mater. Chem. C, 2014, 2, 3846 RSC.
- M. J. Fernandez-Merino, L. Guardia, J. I. Paredes, S. Villar-Rodil, P. Solis-Fernandez, A. Martinez-Alonso and J. M. D. Tascon, J. Phys. Chem. C, 2010, 114, 6426–6432 CAS.
- B. Jeong, S. W. Kim and Y. H. Bae, Adv. Drug Delivery Rev., 2002, 54, 37–51 CrossRef CAS.
- L. Weipeng, L. Shuoqi, F. Wenqian, Q. Junjie, Z. Guoliang, Z. Fengbao and F. Xiaobin, Macromol. Rapid Commun., 2011, 32, 1101–1107 CrossRef PubMed.
- H. Senff and W. Richtering, J. Chem. Phys., 1999, 111, 1705–1711 CrossRef CAS PubMed.
- Z. Sui, X. Zhang, Y. Lei and Y. Luo, Carbon, 2011, 49, 4314–4321 CrossRef CAS PubMed.
- S. R. Raghavan, H. J. Walls and S. A. Khan, Langmuir, 2000, 16, 7920–7930 CrossRef CAS.
- H. Zhou, T. Ni, X. Qing, X. Yue, G. Li and Y. Lu, RSC Adv., 2014, 4, 4134–4139 RSC.
- W. Chen and L. Yan, Nanoscale, 2011, 3, 3132–3137 RSC.
- Q. Yang, Z. Wang and J. Weng, Soft Matter, 2012, 8, 9855–9863 RSC.
- H. Huang, P. Chen, X. Zhang, Y. Lu and W. Zhan, Small, 2013, 9, 1397–1404 CrossRef CAS PubMed.
- B. M. Laing, D. L. Schlueter and T. P. Labuza, J. Food Sci., 1978, 43, 1440–1443 CrossRef PubMed.
- M. Gao, C. K. N. Peh, W. L. Ong and G. W. Ho, RSC Adv., 2013, 3, 13169–13177 RSC.
- Y.-X. Wang, S.-L. Chou, H.-K. Liu and S.-X. Dou, Carbon, 2013, 57, 202–208 CrossRef CAS PubMed.
- X. Zhang, Z. Sui, B. Xu, S. Yue, Y. Luo, W. Zhan and B. Liu, J. Mater. Chem., 2011, 21, 6494–6497 RSC.
| This journal is © The Royal Society of Chemistry 2015 |