DOI:
10.1039/C4RA13221A
(Paper)
RSC Adv., 2015,
5, 1097-1102
Carbazole-based molecular tweezers as platforms for the discrimination of heavy metal ions†
Received
27th October 2014
, Accepted 26th November 2014
First published on 26th November 2014
Abstract
Eight carbazole-based molecular tweezers (CMTs) were designed and synthesized for application in the discrimination of heavy metal ions. All CMT solutions in MeCN were transparent, but exhibited strong deep blue or sky blue fluorescence emission upon UV irradiation. The electronic absorptions of CMTs appeared mainly in the UV region. The CMTs exhibited different fluorescence response patterns upon the addition of various transition metal ions. The principal component analysis (PCA) and linear discriminant analysis (LDA) exhibited clear clustering of data points within the same metal ions and successful dispersion of data clusters. The influence of anionic species on the fluorescence emission of CMTs was almost negligible. Therefore, the chemometric analysis allowed for successful discrimination of 18 heavy metal ions.
Introduction
The release of heavy metal ions into the environment causes serious issues.1–4 Among various pollutants, heavy metal ions cannot be degraded and tend to accumulate in living organisms.5,6 Through the food chain, heavy metal ions can be readily transferred to the human body.6 Heavy metal ions interfere with enzymatic activity and can cause major health problems, such as those associated with arsenic, mercury, cadmium, chromium, lead, and nickel toxicity.7 The most common and effective detection methods employed for heavy metal ions are inductively coupled plasma-mass spectrometry (ICP-MS) and atomic absorption spectrophotometry (AAS).8,9 However, both of these techniques require expensive equipment and time consuming pre-treatment of samples. Therefore, alternative methods which are sensitive, cost-effective and convenient are needed. Within various methods, fluorescence probes may provide powerful tools for small amount of analyte detection due to the high sensitivity. However, selective detection of certain metal ion needed rational design of a target specific recognition moiety. As an alternative way, nonspecific dyes can be arrayed to obtain response patterns as fingerprint of each metal ion.10–22 Statistical treatment of response patterns have been utilized for the discriminations of metal ions,10–13 organic compounds,14,15 sugars,16,17 amino acids,21 and proteins.22
Recently, we reported several carbazole-based molecular tweezers (CMTs),23,24 which make it possible to form stable coordination complexes with several transition metal ions. Due to their unique optical properties, carbazole derivatives are often utilized as a building block of organic light emitting diodes, photorefractive materials, and optical imaging devices.25–28 Because the carbazole moiety has a high fluorescent quantum yield, we envision that carbazole derivatives can be used as a fluorescent probe for the detection of metal ions. The metal coordination possibly influence on the electronic structures of CMTs. Such changes can be directly monitored by fluorescence emission properties. Moreover, the π-conjugated electronic structure of carbazole can be utilized as the building block of conductive polymeric backbone. If the carbazole moieties successfully work as a chemosensor array, we can expand this system into electroactive polymeric sensor array system. In this context, as a proof of concept study, we designed and synthesized eight CMTs and their metal coordination properties were monitored by means of their fluorescence change.
Results and discussion
Design of CMTs
The structures and syntheses of the CMTs are outlined in Scheme 1. Ethynyl, triazole, and pyridyl groups have been introduced to the side group of carbazole moieties to provide metal coordination sites. Using procedures reported in the literature,23,29 acetylene-bearing carbazole (1) and indolocarbazole (5) were prepared as starting materials for the synthesis of molecular tweezers. Cu(I)-catalyzed alkyne–azide click reactions of 1 with benzyl azide and 2-(azidomethyl)pyridine resulted in the production of 2 and 3, respectively. The same click reactions of 5 afforded 6 and 7. Finally, Sonogashira's coupling reactions of 1 and 5 with 2-bromopyridine yielded 4 and 8, respectively. Each CMT was unambiguously characterized by 1H and 13C NMR spectroscopy.
 |
| | Scheme 1 Synthesis of CMTs. Reagents and conditions: (i) benzylazide, CuSO4, sodium L-ascorbate, THF, H2O, 50 °C, 12 h, (ii) 2-(azidomethyl)pyridine, CuSO4, sodium L-ascorbate, THF, H2O, 50 °C, 12 h, (iii) 2-bromopyridine, Pd(PPh3)2Cl2, CuI, Et3N/THF, 60 °C, 24 h. | |
UV/Vis absorptions and fluorescence emissions of CMTs
All CMT solutions in MeCN were transparent, but exhibited strong blue fluorescence emission upon UV irradiation. The electronic absorptions of CMTs appeared mainly in the UV region (Fig. 1). The emission maxima appeared around 370, 395, 410, and 470 nm for 1–3, 5–7, 4, and 8, respectively (Fig. 2). Compared to 1–4, 5–8 exhibited relatively broad emission bands with a sky blue color to the naked eye. The fluorescence quantum yields of the CMTs in MeCN were measured using 9,10-diphenylanthracene (Φ = 0.86 in cyclohexene) as a reference compound.30 As shown in Table 1, all CMTs exhibited high fluorescence quantum yields suitable for application in fluorescence probes. To test the metal binding properties, 19 transition metal ions (5 or 10 equivalents) were added to each CMT solution (10 μM) and the fluorescence emissions were compared under UV light irradiation with a UV handy lamp (λex; 365 nm, Vilber Lourmat, VL-4LC). Fig. 3 shows the fluorescence response of each CMT after 1 h of incubation with 10 and 5 equivalents of various transition metal ions. All CMTs exhibited deep blue (1–4) or sky blue (5–8) fluorescence emissions, but the emission patterns greatly differed from each other. Distinct emission patterns were observed for each combination of the CMTs and transition metal ion additions we tested. In other words, from the fluorescence pattern of each CMT, we can discriminate unknown transition metal species, including the highly toxic heavy metal ions evaluated in the experiments.14 When the amount of transition metal ion additions were reduced to 5 equivalents, the CMT solutions still exhibited similar fluorescence emission patterns, indicating CMTs have high sensitivities against metal ions addition.
 |
| | Fig. 1 UV/Vis absorption spectra of 1–8. | |
 |
| | Fig. 2 Emission spectra of 1–8 upon excitation at 360 nm. | |
Table 1 Quantum yields of CMTs
| Tweezers |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
| MeCN at λex = 325 nm. |
| Φa |
0.49 |
0.36 |
0.53 |
0.06 |
0.48 |
0.42 |
0.53 |
0.12 |
 |
| | Fig. 3 Fluorescence patterns of CMTs (10 μM) in the presence of (a) 10 and (b) 5 equivalents of transition metal ions in MeCN. | |
Chemometric analysis
To verify the usefulness of CMTs for the discrimination of unknown samples, fluorescence response patterns were subjected to chemometric analysis31 using statistical software (SPSS). For this experiment, five independent experiments were carried out and the fluorescence emission intensity changes of each CMT were collected upon the addition of the various metal ions (5 and 10 equivalents; Table S1 and S2,† respectively). First, we performed principal component analysis (PCA),10,18,19 which is a statistical treatment of a multidimensional data set to reduce the data dimension for easy interpretation of the exploratory results. Principal components (PCs) indicate orthogonal eigenvectors that lie in the direction of the maximum variance within the data set. Fig. 4 shows two-dimensional PCA score plots of the fluorescence response of 8 CMTs (10 μM) upon addition of transition metal ions (10 and 5 equivalents), illustrating the scattering of metal ions on a plane formed by the first and second PCs. All data points made a clear clustering in the first two PCs, representing a variance of only 81.1%. The well-dispersed data points in PCA indicate high variability of the fluorescence response with the combinations of the CMTs. By changing the amount of metal ion addition, most data points were not moved except Au3+, Cr3+, Ru3+, Sn2+, and Zn2+, all metal ions maintain their discrimination power at this concentration range.
 |
| | Fig. 4 Two-dimensional PCA score plots of the fluorescence response of 8 CMTs (10 μM) upon addition of (a) 10 and (b) 5 equivalents of transition metal ions. | |
In addition to PCA, linear discriminant analysis (LDA) was carried out to evaluate whether the fluorescence response pattern can be used for identification of the metal ions.11,20 LDA is a statistical method used to differentiate two or more classes of an object or event. It maximizes the between-class variance, but minimizes the within-class variance in a particular data set to maximize the discrimination ability. LDA reduces the size of the training matrix (8 CMTs × 19 metal ions × 5 experiments) and converts them into canonical scores. Fig. 5 shows the two-dimensional LDA plots obtained from 10 equivalents of metal ions addition, which visualize each response pattern onto the plane of the first and second canonical scores. The sum of the first and second canonical scores already occupies 77.8 and 83.5% of the total variation, for 10 and 5 equivalents of metal ion additions, respectively, demonstrating the excellent discrimination ability of the CMTs. If the third canonical score is included in discrimination, the total variation reaches 92.1 and 93.2% for 10 and 5 equivalents of metal ion additions, respectively, indicating the discrimination power was slightly increased by the reduced amount of metal ion additions. Upon the cross-validation routine, in which one random measurement was omitted and the remaining data were used as the training set for the LDA of the random data, all data were assigned an accuracy of 100%.16
 |
| | Fig. 5 Two-dimensional LDA score plots for the discrimination of transition metal ions by using 8 CMTs obtained from (a) 10 and (b) 5 equivalents of metal ions addition. | |
Concentration dependent fluorescence changes
Because the chemometric analysis exhibited high discrimination ability, titration study was conducted for several important metal ions to determine their detection limits. For example, Fig. 6 exhibits spectral change of 1 in MeCN upon successive addition of Hg2+. By addition of Hg2+, the fluorescence emission was completely quenched with linear correlation. Therefore, we can determine the concentration of Hg2+ in μM level. Similarly, the additions of Pb2+, Co2+, Ni2+, Cu2+, Fe3+, and Pd2+ also induced complete fluorescence quenching of CMTs with very high sensitivities (Fig. 7). From the fluorescence responses of each CMT, most of metal ions can be detected in μM level. Typically, Cu2+ ion exhibited the most sensitive response to 2.
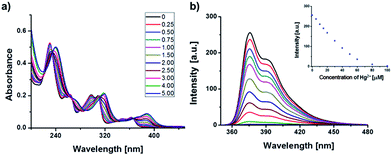 |
| | Fig. 6 Spectral changes of 1 (10 μM) upon successive additions of Hg2+ (0–5 eq.) in MeCN. (a) UV/Vis absorption and (b) fluorescence emission (λex = 303 nm). The inset shows emission change at 376 nm. | |
 |
| | Fig. 7 Fluorescence response of (a) 8 to Pb2+, (b) 2 to Co2+, (c) 3 to Ni2+, (d) 2 to Cu2+, (e) 6 to Fe3+, and (f) 7 to Pd2+ additions. | |
Influence of counter anions
It should be noted that there was no fluorescence change when Li+, Na+, and K+ ions were added to the CMT solutions. In addition, anionic species may bind to the CMTs by hydrogen bonding, which could affect fluorescence responses.32–35 To rule out this possibility, the fluorescence emissions of the CMTs were examined in the presence of common anions as sodium salts. As shown in Fig. 8, no appreciable changes in the fluorescence emissions were observed upon addition of Cl−, AcO−, ClO4−, or NO3−, suggesting that the anion effect on fluorescence response may be negligible.
 |
| | Fig. 8 Fluorescence responses of CMTs (10 μM) upon additions of anions as sodium salts (10 eq.) in MeCN. | |
Experimental
Materials and measurements
All commercially available reagents were reagent grade and used without further purification. Dichloromethane, n-hexane, and tetrahydrofuran (THF) were freshly distilled before each use. Following metal salts were used for the metal ion discrimination experiments; AgClO4·xH2O.
AsCl3, AuCl3, Cd(NO3)2·4H2O, CoCl2·6H2O, CrCl3·6H2O, Cu(OAc)2, Fe(ClO4)2, Fe(ClO4)3, Hg(OAc)2, MnSO4·xH2O, Ni(NO3)2·6H2O, Pb(OAc)2·3H2O, PdCl2, PtCl2, RuCl3, SeCl4, SnCl2, Zn(OAc)2·2H2O. UV/Vis absorption spectra were recorded on a JASCO model V-660 spectrometer. Fluorescence spectra were recorded on a JASCO model FP-6300 spectrometer. 1H and 13C NMR spectra were recorded on a Bruker Avance DPX 400 and DPX 250 spectrometer at 25 °C in CDCl3.
Synthesis
1 and 5 were prepared according to previous reports.23,29
2. To a suspension of 1 (0.10 g, 0.31 mmol) and benzylazide (0.10 g, 0.76 mmol) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and THF (14 mL), sodium L-ascorbate (30 mg, 0.15 mmol) and copper(II) sulfate pentahydrate (12 mg, 0.025 mmol, in 100 μL of water) were added. The reaction mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 2 as pale yellow powder (141 mg, 0.237 mmol, 78%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 11.75 (s, 1H), 8.10 (d, 2H), 7.95 (s, 2H), 7.67 (d, 2H), 7.40 (m, 10H), 5.68 (s, 4H), 1.47 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 148.16, 141.85, 135.84, 134.99, 129.28, 128.85, 128.23, 123.94, 120.37, 119.26, 116.52, 112.76, 54.46, 34.84, 32.20 ppm.
1 mixture of water and THF (14 mL), sodium L-ascorbate (30 mg, 0.15 mmol) and copper(II) sulfate pentahydrate (12 mg, 0.025 mmol, in 100 μL of water) were added. The reaction mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 2 as pale yellow powder (141 mg, 0.237 mmol, 78%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 11.75 (s, 1H), 8.10 (d, 2H), 7.95 (s, 2H), 7.67 (d, 2H), 7.40 (m, 10H), 5.68 (s, 4H), 1.47 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 148.16, 141.85, 135.84, 134.99, 129.28, 128.85, 128.23, 123.94, 120.37, 119.26, 116.52, 112.76, 54.46, 34.84, 32.20 ppm.
3. To a suspension of 1 (0.10 g, 0.31 mmol) and 2-(azidomethyl)pyridine (0.10 g, 0.76 mmol) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and THF (14 mL), sodium L-ascorbate (30 mg, 0.153 mmol) and copper(II) sulfate pentahydrate (12 mg, 0.025 mmol, in 100 μL of water) were added. The reaction mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 3 as white powder (146 mg, 0.246 mmol, 81%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 11.77 (s, 1H), 8.63 (d, 2H), 8.18 (s, 2H), 8.11 (d, 2H), 7.72 (m, 8H), 5.80 (s, 4H), 1.46 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 207.10, 154.65, 149.61, 148.12, 141.76, 137.62, 135.73, 123.84, 123.50, 122.68, 120.33, 119.85, 116.42, 112.65, 55.76, 34.74, 32.10 ppm.
1 mixture of water and THF (14 mL), sodium L-ascorbate (30 mg, 0.153 mmol) and copper(II) sulfate pentahydrate (12 mg, 0.025 mmol, in 100 μL of water) were added. The reaction mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 3 as white powder (146 mg, 0.246 mmol, 81%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 11.77 (s, 1H), 8.63 (d, 2H), 8.18 (s, 2H), 8.11 (d, 2H), 7.72 (m, 8H), 5.80 (s, 4H), 1.46 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 207.10, 154.65, 149.61, 148.12, 141.76, 137.62, 135.73, 123.84, 123.50, 122.68, 120.33, 119.85, 116.42, 112.65, 55.76, 34.74, 32.10 ppm.
4. To a mixture solution of 1 (0.100 g, 0.305 mmol) and 2-bromopyridine (0.087 mL, 0.915 mmol) in dry Et3N/THF (20 mL/10 mL), Pd(PPh3)2Cl2 (7.02 mg, 0.01 mmol) and CuI (9.52 mg, 0.05 mmol) were added. The mixture solution was stirred for 24 h at 60 °C. The solvent was removed under reduced pressure, and the crude mixture was purified by silica gel column with CH2Cl2/hexane as eluent to give 4 (80.79 mg, 0.167 mmol, 55%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 12.53 (s, 1H), 8.67 (d, 2H), 8.20 (s, 2H), 7.80 (m, 8H), 1.46 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 149.30, 143.61, 141.85, 140.54, 136.65, 127.24, 126.58, 123.25, 122.52, 118.30, 104.08, 91.72, 86.37, 34.72, 31.97 ppm.
6. To a suspension of 5 (0.10 g, 0.24 mmol) and benzylazide (0.08 g, 0.60 mmol) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and THF (14 mL), sodium L-ascorbate (25 mg, 0.12 mmol) and copper(II) sulfate pentahydrate (13 mg, 0.05 mmol, in 100 μL of water) were added. The mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 6 as pale green powder (126 mg, 0.184 mmol, 77%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 10.79 (s, 2H), 8.14 (d, 2H), 7.98 (s, 2H), 7.95 (s, 2H), 7.58 (s, 2H), 7.30 (m, 10H), 5.69 (s, 4H), 1.46 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 142.22, 134.80, 129.24, 128.84, 128.09, 126.32, 125.32, 119.24, 119.03, 116.32, 112.50, 111.93, 54.45, 34.77, 32.12, 30.99 ppm.
1 mixture of water and THF (14 mL), sodium L-ascorbate (25 mg, 0.12 mmol) and copper(II) sulfate pentahydrate (13 mg, 0.05 mmol, in 100 μL of water) were added. The mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 6 as pale green powder (126 mg, 0.184 mmol, 77%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 10.79 (s, 2H), 8.14 (d, 2H), 7.98 (s, 2H), 7.95 (s, 2H), 7.58 (s, 2H), 7.30 (m, 10H), 5.69 (s, 4H), 1.46 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 142.22, 134.80, 129.24, 128.84, 128.09, 126.32, 125.32, 119.24, 119.03, 116.32, 112.50, 111.93, 54.45, 34.77, 32.12, 30.99 ppm.
7. To a suspension of 5 (0.10 g, 0.24 mmol) and 2-(azidomethyl)pyridine (0.08 g, 0.60 mmol) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and THF (14 mL), sodium L-ascorbate (25 mg, 0.12 mmol) and copper(II) sulfate pentahydrate (13 mg, 0.05 mmol, in 100 μL of water) were added. The mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 7 as pale green powder (128 mg, 0.187 mmol, 78%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 10.76 (s, 2H), 8.65 (d, 2H), 8.21 (s, 2H), 8.16 (s, 2H), 7.99 (s, 2H), 7.65 (m, 8H), 5.82 (s, 4H), 1.56 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 207.29, 142.48, 137.71, 134.92, 125.51, 123.73, 121.40, 120.20, 119.34, 116.32, 115.74, 112.68, 112.13, 77.58, 77.26, 76.94, 34.95, 32.31, 31.17 ppm.
1 mixture of water and THF (14 mL), sodium L-ascorbate (25 mg, 0.12 mmol) and copper(II) sulfate pentahydrate (13 mg, 0.05 mmol, in 100 μL of water) were added. The mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 7 as pale green powder (128 mg, 0.187 mmol, 78%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 10.76 (s, 2H), 8.65 (d, 2H), 8.21 (s, 2H), 8.16 (s, 2H), 7.99 (s, 2H), 7.65 (m, 8H), 5.82 (s, 4H), 1.56 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 207.29, 142.48, 137.71, 134.92, 125.51, 123.73, 121.40, 120.20, 119.34, 116.32, 115.74, 112.68, 112.13, 77.58, 77.26, 76.94, 34.95, 32.31, 31.17 ppm.
8. To a mixture solution of 5 (0.10 g, 0.24 mmol) and 2-bromopyridine (0.068 mL, 0.72 mmol) in dry Et3N/THF (20 mL/10 mL), Pd(PPh3)2Cl2 (7.02 mg, 0.01 mmol) and CuI (7.62 mg, 0.04 mmol) were added. The mixture solution was stirred for 24 h at 60 °C. The solvent was removed under reduced pressure, and the crude mixture was purified by silica gel column with EtOAc/hexane as eluent to give 8 (71 mg, 0.12 mmol, 52%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 10.56 (s, 2H), 8.55 (d, 2H), 8.19 (s, 2H), 7.73 (s, 2H), 7.52 (m, 8H), 1.50 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 148.82, 142.17, 139.94, 136.46, 126.29, 124.38, 123.85, 122.46, 121.01, 118.27, 112.11, 103.83, 77.34, 77.03, 76.71, 34.83, 32.09 ppm.
Conclusions
We demonstrated that eight CMTs can function as excellent molecular probes for heavy metal ions in MeCN. Characteristic fluorescence response patterns were observed depending on the type of transition metal ion. Although large size of database should be built up for the cross validation, this unique property may be utilized for the determination of toxic heavy metal ions from unknown waste. Unfortunately, we could not provide metal ion detection in aqueous media in this paper due to the poor solubility of CMTs. However, the incorporation of CMT units into conductive polymers may provide more useful electroactive devices. Throughout this proof of concept study, we could successfully prove that the CMTs are powerful metal coordination motifs for the construction of electroactive polymeric sensor arrays.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (no. 2012005565).
Notes and references
- N. Ercal, H. Gurer-Orhan and N. Aykin-Burns, Curr. Top. Med. Chem., 2001, 1, 529–539 CrossRef CAS.
- E. Nieboer and D. H. Richardson, Environ. Pollut., Ser. B, 1980, 1, 3–26 CrossRef CAS.
- L. Järup, Br. Med. Bull., 2003, 68, 167–182 CrossRef PubMed.
- H. Bradl, Heavy Metals in the Environment: Origin, Interaction and Remediation, Academic Press, 2005 Search PubMed.
- A. B. Norberg and H. Persson, Biotechnol. Bioeng., 1984, 26, 239–246 CrossRef CAS PubMed.
- A. Nakajima, T. Horikoshi and T. Sakaguchi, Eur. J. Appl. Microbiol. Biotechnol., 1981, 12, 76–83 CrossRef CAS.
- G. Ysart, P. Miller, M. Croasdale, H. Crews, P. Robb, M. Baxter, C. De L'Argy and N. Harrison, Food Addit. Contam., 2000, 17, 775–786 CrossRef CAS PubMed.
- M. R. Cave, O. Butler, J. M. Cook, M. S. Cresser, L. M. Garden and D. L. Miles, J. Anal. At. Spectrom., 2000, 15, 181–235 RSC.
- M. Ghaedi, H. Tavallali, A. Shokrollahi, M. Zahedi, M. Montazerozohori and M. Soylak, J. Hazard. Mater., 2009, 166, 1441–1448 CrossRef CAS PubMed.
- L. Feng, Y. Zhang, L. Wen, L. Chen, Z. Shen and Y. Guan, Chem.–Eur. J., 2011, 17, 1101–1104 CrossRef CAS PubMed.
- Z. Wang, M. A. Palacios and P. Anzenbacher Jr, Anal. Chem., 2008, 80, 7451–7459 CrossRef CAS PubMed.
- L. H. Yuen, R. M. Franzini, S. Wang, P. Crisalli, V. Singh, W. Jiang and E. T. Kool, Angew. Chem., Int. Ed., 2014, 53, 5361–5365 CrossRef CAS PubMed.
- M. A. Palacios, Z. Wang, V. A. Montes, G. V. Zyryanov and P. Anzenbacher Jr, J. Am. Chem. Soc., 2008, 130, 10307–10314 CrossRef CAS PubMed.
- N. A. Rakow and K. S. Suslick, Nature, 2000, 406, 710–713 CrossRef CAS PubMed.
- M. E. Germain and M. J. Knapp, J. Am. Chem. Soc., 2008, 130, 5422–5423 CrossRef CAS PubMed.
- S. H. Lim, C. J. Musto, E. Park, W. Zhong and K. S. Suslick, Org. Lett., 2008, 10, 4405–4408 CrossRef CAS PubMed.
- A. Schiller, R. A. Wessling and B. Singaram, Angew. Chem., 2007, 119, 6577–6579 CrossRef.
- J. W. Lee, J. S. Lee, M. Kang, A. I. Su and Y. T. Chang, Chem.–Eur. J., 2006, 12, 5691–5696 CrossRef CAS PubMed.
- J.-S. Lee, J. W. Lee and Y.-T. Chang, J. Comb. Chem., 2007, 9, 926–928 CrossRef CAS PubMed.
- L. Baldini, A. J. Wilson, J. Hong and A. D. Hamilton, J. Am. Chem. Soc., 2004, 126, 5656–5657 CrossRef CAS PubMed.
- A. Buryak and K. Severin, J. Am. Chem. Soc., 2005, 127, 3700–3701 CrossRef CAS PubMed.
- O. R. Miranda, C.-C. You, R. Phillips, I.-B. Kim, P. S. Ghosh, U. H. Bunz and V. M. Rotello, J. Am. Chem. Soc., 2007, 129, 9856–9857 CrossRef CAS PubMed.
- H.-C. Gee, C.-H. Lee, Y.-H. Jeong and W.-D. Jang, Chem. Commun., 2011, 47, 11963–11965 RSC.
- H. Yoon, J. M. Lim, H.-C. Gee, C.-H. Lee, Y.-H. Jeong, D. Kim and W.-D. Jang, J. Am. Chem. Soc., 2014, 136, 1672–1679 CrossRef CAS PubMed.
- S. Beaupré, P. L. T. Boudreault and M. Leclerc, Adv. Mater., 2010, 22, E6–E27 CrossRef PubMed.
- S. H. Kim, I. Cho, M. K. Sim, S. Park and S. Y. Park, J. Mater. Chem., 2011, 21, 9139–9148 RSC.
- M. L. Zheng, K. Fujita, W. Q. Chen, N. I. Smith, X. M. Duan and S. Kawata, ChemBioChem, 2011, 12, 52–55 CrossRef CAS PubMed.
- M. Manickam, P. Iqbal, M. Belloni, S. Kumar and J. A. Preece, Isr. J. Chem., 2012, 52, 917–934 CrossRef CAS.
- K. J. Chang, D. Moon, M. S. Lah and K. S. Jeong, Angew. Chem., 2005, 117, 8140–8143 CrossRef.
- J. V. Morris, M. A. Mahaney and J. R. Huber, J. Phys. Chem., 1976, 80, 969–974 CrossRef CAS.
- R. Kramer, Chemometric techniques for quantitative analysis, CRC Press, 1998 Search PubMed.
- D. Curiel, A. Cowley and P. D. Beer, Chem. Commun., 2005, 236–238 RSC.
- J.-m. Suk, M. K. Chae, N.-K. Kim, U.-I. Kim and K.-S. Jeong, Pure Appl. Chem., 2008, 80, 599–608 CrossRef CAS.
- H. Juwarker and K.-S. Jeong, Chem. Soc. Rev., 2010, 39, 3664–3674 RSC.
- K.-J. Chang, B.-N. Kang, M.-H. Lee and K.-S. Jeong, J. Am. Chem. Soc., 2005, 127, 12214–12215 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Raw data of fluorescence intensity used in chemometric analyses. See DOI: 10.1039/c4ra13221a |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and THF (14 mL), sodium L-ascorbate (30 mg, 0.15 mmol) and copper(II) sulfate pentahydrate (12 mg, 0.025 mmol, in 100 μL of water) were added. The reaction mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 2 as pale yellow powder (141 mg, 0.237 mmol, 78%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 11.75 (s, 1H), 8.10 (d, 2H), 7.95 (s, 2H), 7.67 (d, 2H), 7.40 (m, 10H), 5.68 (s, 4H), 1.47 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 148.16, 141.85, 135.84, 134.99, 129.28, 128.85, 128.23, 123.94, 120.37, 119.26, 116.52, 112.76, 54.46, 34.84, 32.20 ppm.
1 mixture of water and THF (14 mL), sodium L-ascorbate (30 mg, 0.15 mmol) and copper(II) sulfate pentahydrate (12 mg, 0.025 mmol, in 100 μL of water) were added. The reaction mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 2 as pale yellow powder (141 mg, 0.237 mmol, 78%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 11.75 (s, 1H), 8.10 (d, 2H), 7.95 (s, 2H), 7.67 (d, 2H), 7.40 (m, 10H), 5.68 (s, 4H), 1.47 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 148.16, 141.85, 135.84, 134.99, 129.28, 128.85, 128.23, 123.94, 120.37, 119.26, 116.52, 112.76, 54.46, 34.84, 32.20 ppm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and THF (14 mL), sodium L-ascorbate (30 mg, 0.153 mmol) and copper(II) sulfate pentahydrate (12 mg, 0.025 mmol, in 100 μL of water) were added. The reaction mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 3 as white powder (146 mg, 0.246 mmol, 81%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 11.77 (s, 1H), 8.63 (d, 2H), 8.18 (s, 2H), 8.11 (d, 2H), 7.72 (m, 8H), 5.80 (s, 4H), 1.46 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 207.10, 154.65, 149.61, 148.12, 141.76, 137.62, 135.73, 123.84, 123.50, 122.68, 120.33, 119.85, 116.42, 112.65, 55.76, 34.74, 32.10 ppm.
1 mixture of water and THF (14 mL), sodium L-ascorbate (30 mg, 0.153 mmol) and copper(II) sulfate pentahydrate (12 mg, 0.025 mmol, in 100 μL of water) were added. The reaction mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 3 as white powder (146 mg, 0.246 mmol, 81%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 11.77 (s, 1H), 8.63 (d, 2H), 8.18 (s, 2H), 8.11 (d, 2H), 7.72 (m, 8H), 5.80 (s, 4H), 1.46 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 207.10, 154.65, 149.61, 148.12, 141.76, 137.62, 135.73, 123.84, 123.50, 122.68, 120.33, 119.85, 116.42, 112.65, 55.76, 34.74, 32.10 ppm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and THF (14 mL), sodium L-ascorbate (25 mg, 0.12 mmol) and copper(II) sulfate pentahydrate (13 mg, 0.05 mmol, in 100 μL of water) were added. The mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 6 as pale green powder (126 mg, 0.184 mmol, 77%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 10.79 (s, 2H), 8.14 (d, 2H), 7.98 (s, 2H), 7.95 (s, 2H), 7.58 (s, 2H), 7.30 (m, 10H), 5.69 (s, 4H), 1.46 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 142.22, 134.80, 129.24, 128.84, 128.09, 126.32, 125.32, 119.24, 119.03, 116.32, 112.50, 111.93, 54.45, 34.77, 32.12, 30.99 ppm.
1 mixture of water and THF (14 mL), sodium L-ascorbate (25 mg, 0.12 mmol) and copper(II) sulfate pentahydrate (13 mg, 0.05 mmol, in 100 μL of water) were added. The mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 6 as pale green powder (126 mg, 0.184 mmol, 77%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 10.79 (s, 2H), 8.14 (d, 2H), 7.98 (s, 2H), 7.95 (s, 2H), 7.58 (s, 2H), 7.30 (m, 10H), 5.69 (s, 4H), 1.46 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 142.22, 134.80, 129.24, 128.84, 128.09, 126.32, 125.32, 119.24, 119.03, 116.32, 112.50, 111.93, 54.45, 34.77, 32.12, 30.99 ppm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and THF (14 mL), sodium L-ascorbate (25 mg, 0.12 mmol) and copper(II) sulfate pentahydrate (13 mg, 0.05 mmol, in 100 μL of water) were added. The mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 7 as pale green powder (128 mg, 0.187 mmol, 78%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 10.76 (s, 2H), 8.65 (d, 2H), 8.21 (s, 2H), 8.16 (s, 2H), 7.99 (s, 2H), 7.65 (m, 8H), 5.82 (s, 4H), 1.56 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 207.29, 142.48, 137.71, 134.92, 125.51, 123.73, 121.40, 120.20, 119.34, 116.32, 115.74, 112.68, 112.13, 77.58, 77.26, 76.94, 34.95, 32.31, 31.17 ppm.
1 mixture of water and THF (14 mL), sodium L-ascorbate (25 mg, 0.12 mmol) and copper(II) sulfate pentahydrate (13 mg, 0.05 mmol, in 100 μL of water) were added. The mixture was stirred vigorously for 12 h at 50°C. The organic layer was separated and dried over MgSO4. After evaporation of the solvent under reduced pressure, the residue was purified using silica gel column with CH2Cl2/hexane as eluent. The residue was recrystallized from hexane to afford 7 as pale green powder (128 mg, 0.187 mmol, 78%): 1H NMR (400 MHz, CDCl3, 25 °C) δ = 10.76 (s, 2H), 8.65 (d, 2H), 8.21 (s, 2H), 8.16 (s, 2H), 7.99 (s, 2H), 7.65 (m, 8H), 5.82 (s, 4H), 1.56 (s, 18H); 13C NMR (100 MHz, CDCl3, 25 °C) δ = 207.29, 142.48, 137.71, 134.92, 125.51, 123.73, 121.40, 120.20, 119.34, 116.32, 115.74, 112.68, 112.13, 77.58, 77.26, 76.94, 34.95, 32.31, 31.17 ppm.



