Electrostatically-induced trajectory switching system on a multi-inlet-multi-outlet superhydrophobic droplet guiding track†
Abstract
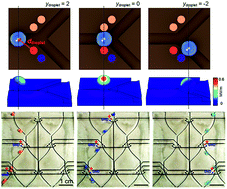
A multi-inlet-multi-outlet (MIMO) superhydrophobic droplet guiding track was demonstrated for water droplet manipulation using an electrostatic force-induced trajectory switching system. Without applying an external electrostatic field, the water droplet rolled along the superhydrophobic guiding track due to its extreme water repellent properties and gravitational force. By applying a DC bias to a capacitor above the guiding track, the trajectory of the water droplet can be easily controlled by the electrostatic attraction. Electrostatically-induced trajectory switching was successfully achieved when the electrostatic and gravitational forces exerted on the water droplet were properly balanced. On a MIMO superhydrophobic droplet guiding track with three inlets and four outlets, the water droplet was guided along the intended trajectory.

 Please wait while we load your content...
Please wait while we load your content...