The efficient and selective biocatalytic oxidation of norisoprenoid and aromatic substrates by CYP101B1 from Novosphingobium aromaticivorans DSM12444†
Abstract
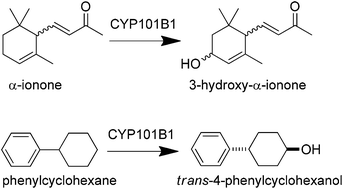
CYP101B1 from Novosphingobium aromaticivorans DSM12444 is a homologue of CYP101A1 (P450cam) from Pseudomonas putida and the CYP101D1, CYP101D2 and CYP101C1 enzymes from the same bacterium. CYP101B1 binds norisoprenoids more tightly than camphor and efficiently hydroxylates substrates in combination with ferredoxin reductase, ArR, and [2Fe–2S] ferredoxin, Arx, electron transfer partners. The norisoprenoids, α-ionone and β-damascone are both oxidised by CYP101B1 with high product formation activity, >500 min−1. α-Ionone oxidation occurred regioselectively at the allylic C3 position while β-damascone was hydroxylated predominantly at C3, 86%, with the main competing minor product arising from oxidation at the allylic C4 position (11%). When incorporated into a whole-cell oxidation system, with ArR and Arx, CYP101B1 is also capable of oxidising the aromatic compound indole. Other aromatic molecules including phenylcyclohexane and p-cymene were tested and both were hydroxylated by CYP101B1. Phenylcyclohexane was selectively oxidised to trans-4-phenylcyclohexanol while p-cymene was hydroxylated at the benzylic carbons to yield a mixture of isopropylbenzyl alcohol and p-α,α-trimethylbenzylalcohol. Trans-4-Phenylcyclohexanol was formed with a product formation rate of 141 min−1 and was five times more active than the oxidation of p-cymene.

 Please wait while we load your content...
Please wait while we load your content...