Preparation of montmorillonite-pillared graphene oxide with increased single- and co-adsorption towards lead ions and methylene blue
Abstract
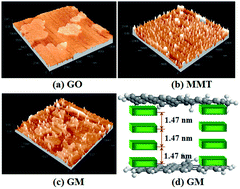
To prevent the aggregation of graphene oxide (GO) during storage or application, montmorillonite (MMT) was used as the modifier, and MMT-pillared GO (GM) was prepared. The features of GM were characterized using Fourier transform infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, energy dispersion X-ray spectrometry, atomic force microscopy and X-ray diffraction measurements. Then, a batch system was applied to study the adsorption behaviors of lead ions (Pb2+) and methylene blue (MB) by GM in single and binary systems. The results showed that GM possessed a higher Brunauer–Emmett–Teller (BET) specific surface area than GO. In a single system, the maximum adsorption capacities of GM for MB and Pb2+ were 350 and 285 mg g−1, respectively. With increasing the storage days, the BET specific surface area and the maximum adsorption capacity of GM remained approximately unchanged, while that of GO dramatically decreased. For a binary system, the presence of MB in water provides additional binding sites for Pb2+, promoting the adsorption of Pb2+ on GM. The presence of Pb2+ in water would compete with MB on GM during the adsorption process, resulting in a decrease in the adsorption of MB. The adsorption results recorded under different conditions indicated that this experiment was capable of the simultaneous removal of Pb2+ and MB using GM as the adsorbent. In addition, the pillared structure of GM greatly enhanced the noncovalent adhesion.

 Please wait while we load your content...
Please wait while we load your content...