Morphological patterns of a growing biological tube in a confined environment with contacting boundary
Abstract
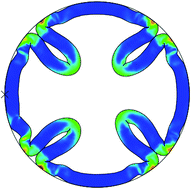
Growing soft tissue with a confined boundary is accompanied by a large strain and stress which lead to instability and the formation of surface wrinkling, folding or creasing. This paper presents the morphological evolution of the growth of a biological tube composed of a neo-Hookean hyperelastic material within a confined environment. Critical growth ratios for the triggering of creases or detachment from the contacting boundary have been investigated both analytically and numerically. Results show that compressive residual stresses induced by confined growth of the tubular tissue can lead to a variety of surface folding patterns which strongly depend on the thickness of the tube. In a thick tube creases begin to form at the inner surface of the tube and in a thin tube the structure detaches from the confining wall. Between these two extremes there is a transitional area wherein the tube starts to crease at first and then detaches from the confining wall. Further modeling reveals that a gap between the tube and the confinement can tune the shape evolution of the growing biological tube. These findings may provide some fundamental understanding to growth modeling of complicated biological phenomena such as cortical folding of the brain and the growth of solid tumors.

 Please wait while we load your content...
Please wait while we load your content...