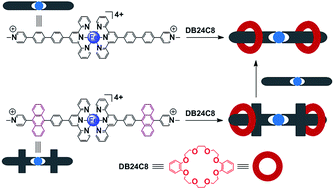
The exchangeable self-assembly behaviour of bis-pseudorotaxanes with metallo-bisviologens (CH3PyTpyFeTpyPyCH34+) and crown ether (Tpy = 2,2′:6′,2′′-terpyridine)†
Abstract
Based on two newly synthesized terpyridine-(1-methyl-pyridiniums) and their metallo-complexes, the exchangeable self-assembly behaviours of metallo-bisviologens with dibenzo-24-crown-8 ether (DB24C8) are comprehensively investigated.

 Please wait while we load your content...
Please wait while we load your content...