Alkylation desulfurization of the C9 fraction over Amberlyst 36 resin
Weiwei Xua and
Yonghong Li*abc
aKey Laboratory for Green Chemical Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: xtx848689@126.com
bNational Engineering Research Center for Distillation Technology, Tianjin 300072, China. E-mail: xtx848689@163.com
cCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China
First published on 26th November 2014
Abstract
The C9 fraction is the by-product of catalytic reforming and in ethylene crackers, and is usually considered as a kind of petroleum resin raw material. Recently, it was studied for use as a gasoline additive to enhance economic benefits. However, the sulfur content of C9 fractions is getting higher. As a result, the C9 fraction alkylation desulfurization process, which consists of weighing down the sulfuric compounds by catalytic alkylation with olefins present in the feed, followed by distillation, has become attractive. In this paper, 2-ethyl-5-methylthiophene, 2,5-dimethylthiophene and 2-n-propylthiophene were selected as model compounds. Firstly, the alkylation reaction of the thiophenic compounds with vinyltoluene was researched over a macroporous sulfonic resin, Amberlyst 36. Then, the octane number of the C9 fraction was measured. It was found that the conversions of the thiophenic compounds could reach above 99%, and the octane number of the C9 fraction was increased. Moreover, the alkylation of thiophenic sulfur could be described as a pseudo-first-order reaction, and the rate constant and activation energy of the alkylation reactions were also calculated.
Introduction
To meet the standards of new environmental legislation and to avoid SOx pollution during fuel combustion, sulfur-containing compounds have to be largely eliminated from fuel.1 As a result, the removal of organosulfur compounds is receiving worldwide attention for environmental protection purposes. Therefore, it is necessary to find a more environmentally friendly method to make refined oil, and new and more economic additives are needed for producing affordable, ultra-clean (ultra-low-sulfur and low-aromatic) transportation fuels and non-road fuels.The C9 fraction is most commonly considered as a kind of petroleum resin raw material, while it has low economic benefits. Recently, the C9 fraction was studied as a gasoline additive, which could enhance its economic benefits, and it has good potential to increase the octane number of gasoline.2 As the by-product of catalytic reforming and in ethylene crackers, a large number of C9 fractions are being produced every year.3 However, the sulfur content of the C9 fraction, which usually has a sulfur content of 250 mg L−1, is getting higher. As a result of these situations, C9 fraction desulfurization is receiving attention due to the increasing stringent regulations on sulfur content.
Conventional hydrotreating technology results in a significant reduction of the octane number due to the saturation of olefins in naphtha from fluid catalytic cracking, which also causes higher hydrogen consumption.4 Consequently, many approaches to deep desulfurization have been proposed.5–10 Among the non-hydrodesulfurization technologies, alkylation desulfurization is rather attractive. This approach consists of increasing the molecular weight of the sulfur compounds using a catalytic alkylation reaction, hence elevating their boiling point, thus allowing sulfur compounds to be easily separated by distillation.11 It is well known that the alkylation of thiophenic compounds occurs through the formation of carbocations,1 and macroporous acid catalysts are needed. Many acidic catalysts either with Brønsted or Lewis acidity were proposed, including various zeolites (β-zeolite, MCM22, USY),12–15 resins (Amberlyst 35),16 and phosphoric acid on kieselguhr (also called solid phosphoric acid),11 etc.
Since the major sulfur species present in the C9 fraction are thiophenic derivatives (C2 and C3 thiophenes),17 we selected 2-ethyl-5-methylthiophene, 2,5-dimethylthiophene and 2-n-propylthiophene as model compounds. Concerning the olefins, the dimerisation product did not remain in the C9 fraction, but it would not reduce the octane number, because it has a far less content in C9 fraction. In this paper, the alkylation of thiophenic compounds in the C9 fraction using Amberlyst 36 resin as catalyst, was carried out in a batch stirred tank reactor. Isoamylene, as the conventional olefin additive, has a good effect on the alkylation desulfurization.16 In order to compare the effect of vinyltoluene and isoamylene on alkylation desulfurization, the alkylation reactions with isoamylene were researched. Furthermore, the kinetics of thiophenic sulfur alkylation with olefins present in the C9 fraction were investigated, including the determination of the reaction rate constant and activation energy.
Experimental
Materials and catalysts
The C9 fraction used in this paper has a sulfur content of 250 mg L−1, with composition: vinyltoluene (5 wt%), α-vinyltoluene (2 wt%), polyene (CnHn,10 wt%), aromatics (65 wt%), and other components (15 wt%). The model feed was self-made with composition: n-decane (95 wt%), thiophenic compound (250 μg g−1) and vinyltoluene (5 wt%), which was representative of non-active C9 components, sulfur compounds and olefins in a real C9 fraction. Isoamylene (+99 wt%, contained 7.2 wt% 2-methyl-1-butene and 92.8 wt% 2-methyl-2-butene) and n-decane (+99.5 wt%) were purchased from Tianjin Guangfu Company and used without any further purification. Vinyltoluene (+99 wt%), 2-ethyl-5-methylthiophene (+99 wt%), 2,5-dimethylthiophene (+99 wt%), and 2-n-propylthiophene (+99 wt%) were purchased from J&K Scientific company and used without any further purification. Amberlyst 36 (Rohm and Hass) was used as the catalyst. According to the supplier, the resin had the following characteristics (see Table 1).| Exchange capacity (mmol g−1 [H+]) | Particle size (mm) | Density (g mL−1) | Average pore size (nm) | Specific area (m2 g−1) | Max temperature (K) |
|---|---|---|---|---|---|
| >5.4 | 0.425–1.180 | 1–1.2 | 24 | 33 | 423 |
Before the experiments, the catalyst was dried at 353 K for 4 h to remove water.
Catalyst evaluation
Reactions were carried out in a batch stirred tank reactor (100 mL), with a stirring rate of 320 rpm. In a typical catalytic experiment for catalyst evaluation, the reactor was charged with 3 g freshly dried catalyst and 60 mL C9 fraction, then it was heated up to the setting temperature. The starting reaction time was taken arbitrarily when the setting temperature was reached. After 1 h of reaction, the autoclave was cooled down to room temperature and the samples were withdrawn to be analyzed.Reaction kinetics analysis
In the experiment, for the reaction process investigation and reaction kinetics analysis, firstly the batch reactor was charged with 3 g freshly dried Amberlyst 36 resin. After the reactor was heated up to the setting temperature, 100 mL model C9 fraction was introduced to the reactor. Meanwhile, the stirrer was adjusted to 320 rpm, in order to ensure the stirrer had no influence on the conversion of the sulfur compounds. The starting reaction time was taken arbitrarily when this temperature was reached. In order to ensure the accuracy of the experiment, ten samples (0.4–0.5 mL) were withdrawn every 25 min during the course of the reaction, and the volume of the samples was negligible.Analysis
The samples were analyzed using a gas chromatograph (FULI 9790, Zhejiang Wenling Company) with a flame ionization detector (FID) and a flame photometric detector (FPD). The FID with a non-polar capillary column (model OV-101, 30 m × 0.25 mm × 0.50 μm) was employed to quantify the hydrocarbons, while the FPD with a capillary column (model OV-101, 60 m × 0.25 mm × 0.50 mL) was utilized for the selective detection of the sulfur compounds at parts-per-million levels in the hydrocarbon mixtures. The total sulfur content of the samples was analyzed by a general coulomb meter (model: DL-2B-EE, Jiangyan Huadong Company), GC-MS (7890A-5975C, Agilent), and an octane number detector (LAB131, Beijing Hightech Lab). For the analysis of the model C9 fraction, the temperature program started at 333 K with a hold time of 5 min, followed by a temperature ramp of 10 K min−1 to 553 K and was then held for 10 min.Results and discussion
Results of the experiments with model sulfur compounds
The C9 fraction was firstly analyzed by GC-FPD and GC-MS. As can be seen from Fig. 1, the sulfur compounds in the C9 fraction were mostly C2–C4 thiophene, and the boiling point of the sulfur compounds was in the temperature range 413 K to 443 K, which was measured by the distillation method. According to the results, 2,5-dimethylthiophene, 2-ethyl-5-methylthiophene, and 2-n-propylthiophene were selected as the sulfur compounds.Alkylation desulfurization of the real C9 fraction over Amberlyst 36
It is well known that the alkylation of thiophenic compounds occurs through the formation of carbocations, and macroporous acid catalysts are needed. Amberlyst 36 is a kind of macroporous sulfonic resin, so Amberlyst 36 can be used as the catalyst. The experiments were carried out at 363 K using the C9 fraction as a feed stock with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 mass ratio of catalyst to C9 fraction. It was found that the sulfur content of the product was 10.21 mg L−1 after one hour. The conversion of thiophenic compounds was 96%, and the octane number of the product was 126RON, while the octane number of the C9 fraction feed was117RON.
30 mass ratio of catalyst to C9 fraction. It was found that the sulfur content of the product was 10.21 mg L−1 after one hour. The conversion of thiophenic compounds was 96%, and the octane number of the product was 126RON, while the octane number of the C9 fraction feed was117RON.
The feasibility of alkylation desulfurization over Amberlyst 36 in the model C9 fraction
To research the feasibility of the reaction process in the model C9 fraction, the reaction was carried out in the model C9 fraction with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.95 mass ratio of Amberlyst 36 to vinyltoluene at 363 K. The results of the experiment are presented in Fig. 2.
1.95 mass ratio of Amberlyst 36 to vinyltoluene at 363 K. The results of the experiment are presented in Fig. 2.
 | ||
| Fig. 2 Diagram of reactant conversion and product formation as a function of time in the model reaction using Amberlyst 36 at 363 K. | ||
It was shown that vinyltoluene oligomerization took place preferably over thiophene alkylation, the reaction rate of thiophene alkylation was higher than vinyltoluene oligomerization, and the content of the oligomers was far less. Moreover, n-decane conversion was close to 0 under all conditions. In this study, side reactions, such as the reaction of oligomers with sulfur compounds, were not detected. Therefore, the alkylation reaction of thiophene compounds with vinyltoluene was the main reaction. This suggests that alkylation desulfurization over Amberlyst 36 in the model C9 fraction is feasible.
Kinetics of alkylation desulfurization of the model C9 fraction over Amberlyst 36
As mentioned above, 2-ethyl-5-methylthiophene, 2,5-dimethylthiophene and 2-n-propylthiophene were chosen to study the kinetics of the alkylation reactions of the C9 fraction in this paper. The experiments were carried out in the model C9 fraction with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio of catalyst to vinyltoluene in the temperature range 343 K to 363 K. Conversions of the thiophenic compounds as a function of reaction time are presented in Fig. 3–5.
1 mass ratio of catalyst to vinyltoluene in the temperature range 343 K to 363 K. Conversions of the thiophenic compounds as a function of reaction time are presented in Fig. 3–5.
 | ||
| Fig. 3 Conversion of 2,5-dimethylthiophene as a function of reaction time at different temperatures. | ||
 | ||
| Fig. 4 Conversion of 2-ethyl-5-methylthiophene as a function of reaction time at different temperatures. | ||
As can be seen from these figures, 2-ethyl-5-methylthiophene, 2,5-dimethylthiophene and 2-n-propylthiophene all had very good alkylation reactivity with vinyltoluene. It was found that the conversion of all three sulfur compounds reached about 99%, but increased slightly with a rise in temperature. In addition, the changing trends of the thiophenic compounds with reaction time showed a similar pattern. Before 75 min reaction time, the conversion of the sulfur compounds was changed dramatically with an increase in temperature. After 100 min, the conversion of the sulfur compounds was more than 99%, and the reactions reached equilibrium quickly. Meanwhile, it could be seen that the conversion of 2-n-propylthiophene was higher than 2-ethyl-5-methylthiophene and 2,5-dimethylthiophene. This indicated that C3-thiophene was more active than C2-thiophene in the model reaction using Amberlyst 36 with vinyltoluene present.
Isoamylene is a good olefin additive for alkylation desulfurization.17 In order to compare the effect of vinyltoluene and isoamylene on alkylation desulfurization, alkylation reactions with isoamylene were carried out. The results (see Table 2) showed that vinyltoluene had a better effect on alkylation of the thiophenic compounds than isoamylene, and after 100 min of the reaction, the reaction rate of carbonation formation of vinyltoluene reached equilibrium. This can be explained by the fact that the reaction rate of carbonation formation of vinyltoluene was more rapid than isoamylene.
| Conversion (%) | ||||||
|---|---|---|---|---|---|---|
| Temperature (K) | 343 | 348 | 353 | 358 | 363 | |
| Vinyltoluene | 2,5-Dimethylthiophene | 93.7 | 94.6 | 95.3 | 96.3 | 97 |
| 2-Ethyl-5-methylthiophene | 95 | 95.8 | 97.1 | 97.3 | 98.4 | |
| 2-n-Propylthiophene | 95.8 | 97.2 | 97.5 | 98.1 | 98.5 | |
| Isoamylene | 2,5-Dimethylthiophene | 68 | 72 | 79 | 81 | 88 |
| 2-Ethyl-5-methylthiophene | 70 | 89 | 90.5 | 94 | 97 | |
| 2-n-Propylthiophene | 80 | 90.5 | 93.1 | 95.7 | 97.9 | |
n-Decane conversion was close to 0 under all conditions during the reaction course. Sulfur compound concentration decreased significantly, which could be explained by the alkylation reaction and the decrease of vinyltoluene concentration. So, the method of using vinyltoluene, which is already present in the C9 fraction, to reduce the sulfur compound concentration of the C9 fraction had a good effect and had no influence on the reaction of n-decane. As the amount of thiophenic compounds in the C9 fraction was far less than vinyltoluene, the amount of vinyltoluene consumed in thiophene alkylation could be neglected.
Apparent reaction kinetics equations
As a bimolecular reaction, the reaction rate of thiophenic sulfur alkylation is theoretically decided by the concentration of olefins and thiophenic sulfur,16 with the reaction rate equation shown in eqn (1). In the experiment, olefins were in much higher molar excess over thiophenic sulfur in the C9 fraction. The concentration of olefins can therefore be assumed to be constant during the entire reaction process.Therefore, the reaction rate equation of thiophenic sulfur alkylation can be simplified as eqn (2)
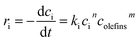 | (1) |
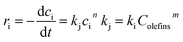 | (2) |
The concentration of olefins can be assumed to be constant during the entire reaction process, and a stirred batch reactor is assumed to determine the reaction rate parameters for the disappearance of thiophenic sulfur. So, i.e. n = 1, the integration of eqn (2) yields eqn (3)
| −ln(1 − Xi) = kit | (3) |
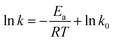 | (4) |
It can be seen from the plots in Fig. 6, −ln(1 − Xi) against time at different temperatures gave straight lines passing through the origin as illustrated. The plots show that the rates of thiophenic sulfur conversion have first-order dependence over the Amberlyst 36 resin with respect to thiophenic sulfur, which is the limiting reactant, while it is of zero-order with respect to olefins which are in large excess.
 | ||
| Fig. 6 Pseudo-first-order kinetic plots with vinyltoluene present: (a) 2,5-dimethylthiophene; (b) 2-ethyl-5-methylthiophene; (c) 2-n-propylthiophene. | ||
From the plots in Fig. 6, ki (the reaction rate constant) could be evaluated, with the results listed in Table 4. The representative Arrhenius plots for the reaction rate constant employing the Arrhenius equation are shown in Fig. 7. It can be seen from Table 3 that the fit of the experimental values to the Arrhenius equation is rather perfect, and the values of Ea and k0 derived from these plots are: 17.03 kJ mol−1 and 1.25 × 104 h−1 for 2,5-dimethylthiophene, 16.55 kJ mol−1 and 1.19 × 104 h−1 for 2-ethyl-5-methylthiophene, 12.93 kJ mol−1 and 2.93 × 103 h−1 for 2-n-propylthiophene.
| Ea (kJ mol−1) | k0 (h−1) | |
|---|---|---|
| 2,5-Dimethylthiophene | 17.03 | 1.25 × 103 |
| 2-Ethyl-5-methylthiophene | 16.55 | 1.19 × 103 |
| 2-n-Propylthiophene | 12.93 | 2.93 × 102 |
| Temperature (K) | 343 | 348 | 353 | 358 | 363 |
|---|---|---|---|---|---|
| 2,5-Dimethylthiophene | 1.16 | 1.28 | 1.36 | 1.50 | 1.61 |
| 2-Ethyl-5-methylthiophene | 1.31 | 1.40 | 1.55 | 1.63 | 1.87 |
| 2-n-Propylthiophene | 1.52 | 1.64 | 1.73 | 1.85 | 1.98 |
Kinetics parameters of different model sulfur compounds
As can be seen from Table 4, the reaction rate constants of 2-n-propylthiophene are larger than for the others under the same conditions. Furthermore, the reaction rate constant increased with the rise in temperature, which indicates that high temperatures could promote the effect of alkylation reactions of thiophenic sulfur. This can be explained by the following two aspects. Firstly, as the reaction rate of carbonation formation grows with the increase in temperature, alkylation reactions of thiophenic sulfur have longer reaction times at high temperatures. Secondly, as an exothermic reaction, the reaction equilibrium constant decreases with increasing temperature,16 so more olefins remain in the system, which would increase the reaction rate of alkylation. The three thiophenic compounds show similar orders for the activation energy and pre-exponential factor: 2,5-dimethylthiophene > 2-ethyl-5-methylthiophene ≈ 2-n-propylthiophene, which indicates that the olefin could enfold the thiophene ring and combine with the α-position of the S atom.The kinetics of the alkylation mechanism follows the Eley–Rideal law11 and the reaction intermediate seems to be an ester of sulfonic acid. In other words, only the olefin (vinyltoluene) is adsorbed on the Amberlyst 36 resin to form a carbocation through an ester of sulfonic acid. Then, the thiophenic molecule in the liquid phase reacts with this ester to form a monoalkyl, which will next be desorbed by giving back a proton to the surface.
Conclusions
Through the experiments in the model C9 fraction, it was found that 2-ethyl-5-methylthiophene, 2,5-dimethylthiophene and 2-n-propylthiophene all had very good alkylation reactivity with vinyltoluene. Conversions of thiophenic compounds were all close to 99% after 150 min of the reaction, and the octane number of the C9 fraction was increased by the alkylation reaction, which suggests a new way to reduce the sulfur content in the C9 fraction. However, 2-n-propylthiophene had higher conversions than 2-ethyl-5-methylthiophene and 2,5-dimethylthiophene, and the alkylation reaction rate of 2-n-propylthiophene reached equilibrium after 125 min of the reaction, which was more rapid than 2-ethyl-5-methylthiophene and 2,5-dimethylthiophene. This indicated that C3-thiophene was more active than C2-thiophene in the model reaction using Amberlyst 36 with vinyltoluene present. This can be explained by the fact that the alkylation reaction is a kind of electrophilic substitution reaction which mainly occurs at the α-position of the thiophenic compound. As methyl, ethyl and propyl are all electron-donating groups which can strengthen the electronegativity of the α-position of the thiophenic ring, on the contrary, vinyltoluene is a kind of electrophile. So, the electronegativity can make the alkylation reaction of thiophenic compounds with vinyltoluene happen more easily. As we know, the effect of the electron-donating groups is in the order: propyl > ethyl > methyl. Moreover, 2,5-dimethylthiophene and 2-ethyl-5-methylthiophene both have two α-branched chains, so the alkylation reaction must happen at the β-position of the thiophenic ring, which is more difficult than the reaction which happens at the α-position of the thiophenic ring. So, C3-thiophene was more active than C2-thiophene.Moreover, the kinetics parameters of the different model sulfur compounds were calculated, and the alkylation of the three thiophenic compounds in the model C9 fraction shows the kinetic characteristics of an apparent pseudo-first-order reaction, assuming that the concentration of olefins is constant.
Acknowledgements
We gratefully acknowledge the financial support by the Program of Universities’ Innovative Research Teams (no. IRT0936).Notes and references
- M. Breysse, G. Djega-Mariadassou and S. Pessayre, et al. Deep desulfurization: reactions, catalysts and technological challenges, Catal. Today, 2003, 84, 129–138 CrossRef CAS
.
- H. Shapiro, E. G. De Witt and J. E. Brown, US 2,839,552, CA, 17 June 1958, vol. 52, p. 17286
.
- Y. V. Dumskii, G. M. Butov and G. F. Cherednikova, et al. Synthesis of petroleum polymer resins by initiated oligomerization of the C8/C9 gasoline pyrolysis fraction, Pet. Chem., 2014, 54, 69–71 CrossRef CAS
.
- R. Shafi and G. J. Hutchings, Hydrodesulfurization of hindered dibenzothiophenes: an overview, Catal. Today, 2000, 59, 423–442 CrossRef CAS
.
- N. A. Collins and J. C. Trewella, Alkylation process for desulfurization of gasoline, U.S. Patent 5, 599, 441, 4 February 1997
.
- C. Song and X. Ma, New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization, Appl. Catal., B, 2003, 41, 207–238 CrossRef CAS
.
- J. Zhang, A. Wang and X. Li, et al. Oxidative desulfurization of dibenzothiophene and diesel over H3PMo12O40, J. Catal., 2011, 279, 269–275 CrossRef CAS PubMed
.
- C. Yansheng, L. Changping and J. Qingzhu, et al. Desulfurization by oxidation combined with extraction using acidic room-temperature ionic liquids, Green Chem., 2011, 13, 1224–1229 RSC
.
- A. R. Hansmeier, G. W. Meindersma and A. B. de Haan, Desulfurization and denitrogenation of gasoline and diesel fuels by means of ionic liquids, Green Chem., 2011, 13, 1907–1913 RSC
.
- K. Kedra-Królik, M. Fabrice and J. N. Jaubert, Extraction of thiophene or pyridine from n-heptane using ionic liquids. Gasoline and diesel desulfurization, Ind. Eng. Chem. Res., 2011, 50, 2296–2306 CrossRef
.
- V. Bellière, C. Lorentz and C. Geantet, et al. Kinetics and mechanism of liquid-phase alkylation of 3-methylthiophene with 2-methyl-2-butene over a solid phosphoric acid, Appl. Catal., B, 2006, 64, 254–261 CrossRef PubMed
.
- L. Hu, Z. Zhang and S. Xie, et al. Effect of grain size of zeolite Y on its catalytic performance in olefin alkylation thiophenic sulfur process, Catal. Commun., 2009, 10, 900–904 CrossRef CAS PubMed
.
- Y. Liu, B. Yang and C. Yi, et al. Kinetics study of 3-methylthiophene alkylation with isobutylene catalyzed by NKC-9 ion exchange resin, Ind. Eng. Chem. Res., 2011, 50, 9609–9616 CrossRef CAS
.
- Z. Zhang, X. Li and L. Zhang, et al. Modification of HY zeolite by fluorine and its influence on olefin 3alkylation thiophenic sulfur in gasoline, Asia-Pac. J. Chem. Eng., 2008, 3, 503–508 CrossRef CAS
.
- L. F. Isernia, FTIR study of the relation, between extra-framework aluminium species and the adsorbed molecular water, and its effect on the acidity in ZSM-5 steamed zeolite, Mater. Res., 2013, 16, 792–802 CrossRef CAS PubMed
.
- B. Guo, R. Wang and Y. Li, Gasoline alkylation desulfurization over Amberlyst 35 resin: Influence of methanol and apparent reaction kinetics, Fuel, 2011, 90, 713–718 CrossRef CAS PubMed
.
- G. Sun and G. Liu, Analysis of the C9 Fraction by GC-MS and GC-FTIR, J. Instrum. Anal., 2004, 23, 289–292 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |



