Fully aromatic naphthalene-based sulfonated poly(arylene ether ketone)s with flexible sulfoalkyl groups as polymer electrolyte membranes†
Baolong Wanga,
Zhenzhen Caib,
Na Zhanga,
Bin Zhangb,
Duo Qia,
Chengji Zhao*a and
Hui Na*a
aAlan G. MacDiarmid Institute, College of Chemistry, Jilin University, Changchun 130012, PR China. E-mail: zhaochengji@jlu.edu.cn; huina@jlu.edu.cn; Fax: +86-431-85168870; Tel: +86-431-85168870
bState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, China
First published on 24th November 2014
Abstract
A series of sulfonated naphthalene-based poly (arylene ether ketone)s (SNPAEK-xx) with pendant sulfoalkyl groups were prepared by polycondensation of 1,5-bis(4-fluorobenzoyl)-2,6-dimethoxynaphthalene and o-methylhydroquinone, followed by a demethylation and sulfobutylation reaction. The sulfonate degree of SNPAEK-xx could be controlled easily by adjusting the ratio of 1,4-butane sultone to the hydroxyl content in the demethylated polymers. Flexible and tough membranes with reasonably high mechanical strength were prepared. SNPAEK-xx membranes showed a high ionic exchange capacity (IEC) in the range of 1.13 to 2.27 mequiv. g−1, and the highest proton conductivity of 0.191 S cm−1 at 80 °C. They exhibited low methanol permeability in the range of 1.25–10.22 × 10−7 cm2 s−1, which was much lower than that of Nafion 117. Transmission electron microscopy analysis of SNPAEK-xx revealed that they had a more obvious phase separated structure between the hydrophilic side chain and hydrophobic fully aromatic domains at a higher IEC. Combining their high thermal and mechanical stability, high selectivity, lower water swelling ratio, SNPAEK-xx membranes could be promising materials as alternative to Nafion membranes for direct methanol fuel cell applications.
Introduction
Polymer electrolyte fuel cells (PEFCs) are gaining more and more attention due to their high durability and potentially lower cost for stationary and portable power applications.1–3 The polymer electrolyte membrane (PEM), which separates two electrodes and transports protons, plays a dominant role in a PEFC system. PEMs usually require a high proton conductivity, low methanol permeability and high mechanical strength. Perfluorosulfonic acid polymers, such as Nafion produced by DuPont, which meet the above crucial requirements, are considered the state-of-the-art PEM materials. However, their drawbacks, such as high cost, high methanol permeability, and relatively low operation temperature (<80 °C), restricted their practical applications in PEFCs.4,5 Thus, the development of well-balanced alternatives to perfluorosulfonic acid polymers is a major challenge.Hydrocarbon-based aromatic polymers have been extensively investigated as candidate PEM materials because of their lower production cost, high-temperature stability and good mechanical strength. These materials developed by many research groups include sulfonated poly(arylene ether ketone),6–8 sulfonated poly(arylene ether sulfone),9–14 sulfonated polyimides,15–17 sulfonated poly(arylene ether nitrile),18,19 and sulfonated polybenzimidazole.20,21 Generally, the sulfonated groups in these polymers are located on the main chain and directly attached to the rigid backbone of polymers; therefore, these polymers are unable to form distinct phase-separated structures and have more dead-end channels compared to Nafion. As a result, these main-chain-type sulfonated polymers only achieve sufficient conductivities comparable with Nafion at much higher ion exchange capacity (IEC). However, high IECs usually lead to excess water swelling at elevated temperatures, thereby causing large dimensional variations and failing to afford enough mechanical strength. To balance the tradeoff between dimensional stability and proton conductivity of aromatic polymers is crucial for improving PEM performance by careful structural design. One of the most promising approaches to solve these shortages of main-chain-type aromatic polymers is to simulate the chemical structure of Nafion and to separate the hydrophilic sulfonic acid domain from the hydrophobic main-chain domain by locating these ionic groups far away on the pendent side chains to restrict the water swelling of the sulfonated hydrophilic domain.22–25 A variety of side-chain-type sulfonated polymers have been prepared by the direct copolymerization method or by chemical grafting the pendants onto polymers.26,27 In general, they demonstrated a comparatively better balance between proton conductivity and water dimensional swelling than main-chain-type sulfonated polymers. For the direct copolymerization method, Pang et al. synthesized a new sulfonated monomer with sulfonic acid groups on flexible aliphatic chains and prepared the sulfonated poly(arylene ether)s with pendant sulfoalkyl groups by a direct copolymerization method.28 The sulfonated monomer was prepared by an anhydrous aluminum chloride-catalyzed Friedel–Crafts acylation of 1-bromo-3-phenylpropane with 2,6-difluorobenzoyl chloride and subsequently sulfonated with Na2SO3. The generated membranes exhibited high proton conductivities and a very low swelling ratio at high temperature. In addition, some side-chain-type sulfonted polyimides had been prepared from sulfonated diamine monomers by direct copolymerization.29,30 These polymers also displayed advantageous conductivity and membrane hydrodynamic properties compared to most of main-chain-type sulfonated polymers. Besides the direct copolymerization method, the grafting of sulfonic acid groups onto polymer side chains is another efficient method to prepare side-chain-type sulfonated aromatic polymers. Jannasch and coworkers prepared branched sulfonated polysulfones via lithiation and sulfoalkylation of polysulfone.31 Ding and coworkers synthesized comb-shaped polymers that had rigid aromatic main chains and flexible side chains.32 In our previous work, we reported a series of novel side-chain-type naphthalene-based sulfonated poly(arylene ether ketone) copolymers (SNPAEKs) by a demethylation and sulfobutylation method. The highest conductivity of 0.179 S cm−1 was obtained for SNPAEKs (IEC = 1.82 mequiv. g−1) at hydrated state at 80 °C, which is higher than that of Nafion 117 (0.149 S cm−1) at the same condition. Furthermore, the introduction of naphthalene moiety into SNPAEKs increased the free volume and the stiffness of polymer chain and restricted the water swelling, thereby improving the in-water dimensional stability and mechanical properties as PEM materials. However, they had relatively low IEC values in the range of 0.99–1.82 mequiv. g−1, which hindered the further improvement in the proton conductivity or related properties. Because of the highly hydrophobic structure of naphthalene moieties in the main chain, achieving PEMs with higher IEC values, higher proton conductivity, but acceptable dimensional stability is also possible. Moreover, increasing IEC enhances the difference between hydrophilic sulfonic acid domains and hydrophobic domains, thus inducing more distinct phase-separated morphology.
In this study, the fully aromatic naphthalene-based sulfonated poly(arylene ether ketone)s with high density of alkylsulfonic acid groups were successfully prepared. The IEC values for this novel series of side-chain-type SNPAEKs can reach up to 2.27 mequiv. g−1, accompanying with high proton conductivities of 0.094 S cm−1 at 25 °C and 0.191 S cm−1 at 80 °C, respectively. Fortunately, the fully aromatic structures in the polymer main chain suppressed the water swelling and maintained dimensional stability in spite of high IEC values. The morphology and the detailed properties of PEMs, such as water uptake, swelling ratio, proton conductivity, thermal and mechanical properties, were also reported here. Moreover, we designed a new precise and fast measurement method to determine the methanol permeability of PEMs, using 1H NMR to calculate the methanol concentration in the permeation cell as a function of time, which is different from the conventional calculated method by using meteorological chromatography or by using refractive index detector. Due to the fully aromatic structure and naphthalene moieties in the main chain, SNPAEKs with high IEC values still have low methanol permeability, low swelling ratio and high selectivity, which meet the fundamental requirements for applications in direct methanol fuel cells (DMFCs).
Experimental
Materials
1,5-Bis(4-fluorobenzoyl)-2,6-dimethoxynaphthalene (DMNF) was synthesized in our lab according to our previous report.3 2,6-Dimethoxynaphthalene was obtained from Dalian Jinzhou Chemical plant. 4-Fluorobenzoyl chloride and 1,4-butane sultone were purchased from Sigma-Aldrich. Boron tribromide (BBr3), o-methylhydroquinone and NaH (60%) were purchased from Aladdin Scientific Co. Ltd. Shanghai. The solvents, N,N-dimethylacetamide (DMAc), dimethylsulfoxide (DMSO), and N-methyl-2-pyrrolidone (NMP) were vacuum-distilled before used. Other reagents were commercially available grade and used without further purification.Synthesis of naphthalene-based poly(arylene ether ketone) containing methoxyl groups
A typical synthetic procedure for the preparation of MM-PAEK is described as follows. In a 250 mL three-necked flask equipped with a mechanical stirrer, a Dean–Stark trap and a nitrogen inlet, DMNF (8.64 g, 0.02 mol), o-methylhydroquinone (2.48 g, 0.02 mol), K2CO3 (2.76 g, 0.02 mol), NMP (50 mL) and toluene (15 mL) were placed. The mixture was slowly heated to 120 °C and kept at this temperature for about 3 h to remove the water produced by reaction. The temperature was slowly raised to 180 °C and maintained for another 12 h. The resulting viscous solution was cooled down to room temperature, precipitated into a large excess of deionized water with vigorous stirring. The resulting fibrous polymer was filtered and washed thoroughly with deionized water several times and dried under vacuum at 120 °C for 24 h. The polymer was denoted as MM-PAEK.Synthesis of naphthalene-based poly(arylene ether ketone) containing phenolic hydroxyl groups
The methoxy groups in MM-PAEK polymer were converted into hydroxyl functionalities according to a modified procedure of demethylation reaction as McOmie et al. reported.33 The typical demethylation reaction is described as follow: MM-PAEK (1.0 g) was dissolved in 20 mL refined CH2Cl2. The solution was cooled down using liquid N2, and 1 M BBr3 solution of CH2Cl2 12 mL was added dropwise. Then the reaction was increased to room temperature and stirred for another 12 h. Then the mixture was stripped out with 100 mL ice-water, and then washed with ethanol and deionized water several times. The resulted polymer (HO-PAEK) was dried under vacuum at 120 °C for 24 h to remove the remaining solvents.Synthesis of naphthalene-based poly(arylene ether ketone) with pendent sulfoalkyl groups
The sulfobutylation reaction is described as follows: HO-PAEK (1.0 g)polymer was dissolved in 20 mL anhydrous DMSO at room temperature for 24 h in oil bath, and then heated to 60 °C under N2 atmosphere. After HO-PAEK was completely dissolved in DMSO, NaH (0.30 g) solid was added quickly and reacted for another 30 min. 0.80 mL 1,4-butane sultone was subsequently added to the mixture, and kept stirring at 60 °C for 30 min. Then the reaction was heated at 120 °C for 24 h in oil bath, and precipitated into anhydrous acetone. The obtained pale yellow solid powder was obtained by filtered, washed with acetone and deionized water for several times, and dried in vacuum oven at 120 °C for 24 h to remove the remained solvents. The resulted polymers were nominated as SNPAEK-xx, where xx stood for the contents of flexible sulfoalkyl side chain of every repeat unit.Membrane formation and proton exchange
Membranes were prepared by casting SNPAEK-xx (in their sodium form) solution in DMSO onto the glass plates and dried at 100 °C for 24 h. The membranes were then peeled off from the substrates, and immersed in 1 M HCl at room temperature for 24 h to thoroughly convert into acid form. In order to remove any remaining solvent, the membranes were dried under vacuum at 120 °C for another 48 h.Measurements
1H NMR spectra were recorded on a Bruker 510 instrument using CDCl3 and/or DMSO-d6 as solvent and tetramethyl silane (TMS) as an internal standard. The inherent viscosities of the obtained polymers were determined with a concentration of 0.5 g dL−1 polymer in DMSO and/or NMP at 30 °C by using Ubbelohde capillary viscometer. The glass transition temperatures (Tg) of polymers were determined by using differential scanning calorimeter (DSC) measurement performed on a TA DSC Q20 instrument at heating and cooling rate of 10 °C min−1 in a temperature range of 100–300 °C under nitrogen. The thermo gravimetric analyses (TGA) were performed on a Pyris 1 thermo gravimetric analyzer (Perkin-Elmer) from 100 to 700 °C at a heating rate of 10 °C min−1 under nitrogen atmosphere. The mechanical properties of thin membranes were evaluated at room temperature with a universal testing instrument (SHIMADZU AG-I 1KN) at a speed of 2 mm min−1. TEM images were obtained with JEM-2100F electron microscope operating at 200 kV. Before test, the polymers were first converted into Ag+ form by immersing the polymers in AgNO3 solutions for 24 h. The SNPAEK-Ag in DMSO solutions was then casted onto copper grids for TEM use.Ion exchange capacity, water uptake and swelling ratio
The IEC and water uptake of membranes were determined by the method similar to that reported previously.8 The dimensional changes of these membranes were measured in the vertical and plane direction, which were characterized by
 | (1) |
 | (2) |
Proton conductivity
The in-plane proton conductivity (σ) was measured by a four-electrode AC impedance method from 0.1 Hz to 100 KHz, using Princeton Applied Research Model 2273 potentiostat/galvanostat/FRA. All the membranes were cut into 1 cm × 4 cm pieces and the thicknesses were measured. The membranes were dipped in deionized water for at least 24 h before analysis. A sheet of membrane was placed in the test cell, which was immersed in water with 100% relative humidity at desired temperature. The proton conductivity (σ) was calculated from the following relationship.
 | (3) |
Methanol permeability
Methanol permeability through the membranes was measured using a homemade two-chamber diffusion cell, which consists of two compartments separated by a vertical membrane. One compartment was filled with 10 M methanol aqueous solution (donor) and the other with deionized water (receptor). Each liquid compartment was stirred by a magnetic stirrer to ensure uniformity. Methanol concentration (measured in mol L−1) in the donor compartment was held at a constant value, and the methanol concentration in the receptor compartment as a function of time was measured by 1H NMR. For this purpose, 1 mL methanol solution was collected from the receptor compartment at a desired time by using 1 mL pipette and transferred into a standard 5 mm NMR tube with DMSO-d6 as solvent. Then 10 µL HPLC THF was also added to the NMR tube by using a 10 µL microinjector as standard substance. After mixing them by ultrasonic, 1H NMR measurements were performed on a Bruker 510 instrument. The methanol concentration (CB) was then calculated from integral area ratio of the signals of methanol and HPLC THF in the obtained 1H NMR spectra. Last, the methanol permeability of SNPAEK membranes was calculated as follows:| P = DK | (4) |
 | (5) |
Results and discussion
Synthesis and characterization of polymers
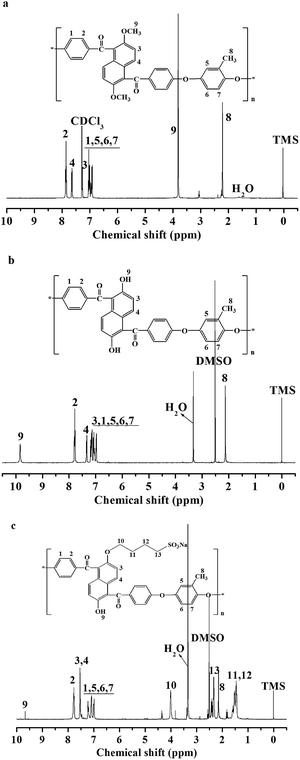
According to our previous work, a naphthalene-based monomer DMNF was prepared by an anhydrous ferric chloride catalyzed Friedel–Crafts acylation of 2,6-dimethoxynaphthalene with 4-fluorobenzoylchloride. Then, as shown in Scheme 1, the naphthalene-based poly(arylene ether ketone) polymer containing methoxy groups (MM-PAEK) was synthesized by a standard K2CO3-catalyzed aromatic nucleophilic substitution polycondensation of DMNF and o-methylhydroquinone. The polymerization reaction proceeded smoothly to high molecular weight as no evident cross-linking was found even at the temperature higher than 180 °C. MM-PAEK exhibited high intrinsic viscosity (1.0 g dL−1), which indicated the resulted polymer had high molecular weight.As shown in Scheme 2, the demethylation of MM-PAEK polymer to the reactive hydroxyl-containing HO-PAEK for grafting was conducted at room temperature in dichloromethane using BBr3. HO-PAEK was precipitated during the reaction due to the polar nature of phenolic hydroxyl groups. Fig. 1 shows the 1H NMR spectra of MM-PAEK and HO-PAEK. The characteristic peak at 3.78 ppm attributing to the methoxy protons in the initial MM-PAEK disappeared in HO-PAEK, while the new peak at around 9.83 ppm corresponding to the proton of –OH group was observed in the spectrum of HO-PAEK. The results indicated that the demethylation reaction had completely proceeded. Finally, the sulfobutyl groups were grafted onto HO-PAEK by a nucleophilic ring opening reaction with 1,4-butane sultone in the presence of NaH. SNPAEK-xx with different contents of sulfobutyl groups were obtained by adjusting the ratio of 1,4-butane sultone to the hydroxyl content in HO-PAEK.
Fig. 1 also shows a representative 1H NMR spectrum of SNPAEK-xx, which has the signal assignments consistent with the designed molecular structure. As expected, the hydroxyl protons at around 9.83 ppm in HO-PAEK almost disappeared, while new signals for the methylene protons were observed at 3.97 ppm, 2.33 ppm, 1.52 ppm, 1.45 ppm, which demonstrated that the sulfonic acid groups were introduced to polymers by sulfobutylation reaction. By calculating integral area ratio of 3.97 ppm (Hm) and 7.78 ppm (Ha), the degree of sulfonation (DS) of SNPAEK ranging from 0.65 to 1.60 was obtained according to the following equation.
 | (6) |
Mechanical and thermal properties
As shown in Table 1, SNPAEK-xx membranes showed much higher inherent viscosities than that of the corresponding MM-PAEK because of the grafting of sulfobutyl groups, which enhanced intermolecular associations and gave a stronger interaction between polymer chains. The high viscosities also indicated that there was no obvious polymer decomposition or crosslinking occurred under basic conditions during the sulfobutylation reaction.| Samples | ηinh (dL g−1) | Ambient condition | Fully hydrated state | ||||
|---|---|---|---|---|---|---|---|
| Tensile strength (MPa) | Young's modulus (GPa) | Elongation at break (%) | Tensile strength (MPa) | Young's modulus (GPa) | Elongation at break (%) | ||
| a The polymer was dissolved in NMP.b The polymer was dissolved in DMSO.c The mechanical properties of Nafion 117 are adapted from ref. 34. | |||||||
| MM-PAEKa | 1.00 | 63.7 ± 4.0 | 1.08 ± 0.09 | 7.4 ± 0.7 | — | — | — |
| SNPAEKb-0.65 | 1.49 | 67.0 ± 4.0 | 1.76 ± 0.07 | 14.8 ± 3.8 | 62.3 ± 3.5 | 0.29 ± 0.03 | 33.8 ± 4.6 |
| SNPAEKb-1.00 | 1.06 | 52.0 ± 2.0 | 1.39 ± 0.04 | 17.3 ± 7.9 | 45.5 ± 1.0 | 0.26 ± 0.02 | 31.1 ± 2.7 |
| SNPAEKb-1.30 | 1.67 | 42.6 ± 1.6 | 1.09 ± 0.07 | 40.3 ± 4.5 | 45.0 ± 3.4 | 0.35 ± 0.03 | 35.6 ± 6.4 |
| SNPAEKb-1.47 | 1.70 | 32.0 ± 2.2 | 0.84 ± 0.04 | 55.0 ± 5.3 | 44.0 ± 2.5 | 0.53 ± 0.04 | 33.2 ± 3.7 |
| SNPAEKb-1.60 | 2.03 | 35.6 ± 2.1 | 0.88 ± 0.02 | 77.7 ± 7.5 | 35.1 ± 1.7 | 0.16 ± 0.02 | 40.2 ± 1.7 |
| Nafion 117c | — | 29.4c | 0.357c | 270c | — | — | — |
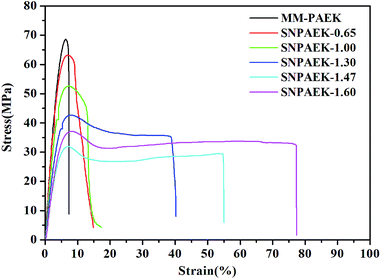
Typical tensile stress–strain curves of all the SNPAEK-xx membranes are shown in Fig. 2 under ambient condition, and the results are also listed in Table 1. For the MM-PAEK membranes containing naphthalene moieties, it exhibited a reasonably high mechanical strength with a tensile strength at maximum load 63.7 MPa, Young's modulus of 1.08 GPa, and elongation at break of 7.4%. After sulfobultylation, SNPAEK-xx membranes still showed better mechanical properties with high tensile strength ranging from 32.0 to 67.0 MPa, and Young's modulus ranging from 0.84 to 1.76 GPa than Nafion 117 which has a tensile strength of 29.4 MPa and a Young's modulus of 0.357 GPa, respectively. Furthermore, it can be seen from Fig. 2 that SNPAEK-xx membranes exhibited a decreasing tendency in tensile strength and Young's modulus with the sulfobutyl content increasing. It's worth to mention that the elongation at break of SNPAEK-xx significantly increased from 14.8% to 77.7% as the sulfobutyl content increased from 0.65 to 1.60. This could be attributed to the introduction of flexible aliphatic side-chain segments, which acted as a plasticizer to the high chain rigidity of aromatic polymer backbone. As shown in Table 1, compared with ambient condition, fully hydrated SNPAEK-xx membranes also showed good mechanical properties with tensile strength ranging from 35.1 to 62.3 MPa, and Young's modulus ranging from 0.16 to 0.53 GPa, respectively. As the water molecule in the hydrated membranes acted as the plasticizer, all these SNPAEK-xx membranes exhibited high elongation at break ranging from 31.1 to 40.2%. All the results indicated that these membranes were strong and tough for potential use as PEM materials in fuel cells.
Due to the high chain rigidity of fully aromatic structures, MM-PAEK containing methoxyl groups and HO-PAEK containing hydroxyl groups exhibited the high glass transition temperatures (Tg) of 239 °C and 227 °C, respectively (Fig. 3). However, no obvious glass transition could be observed below decomposition temperature for SNPAEK polymers containing sulfobutyl groups. To further investigate the thermal stabilities of SNPAEK polymers, TGA analysis was carried out under nitrogen. Fig. 4 shows the TGA and their derivative curves (DTG) of SNPAEK polymers with different sulfobutyl groups. All the polymers displayed a two-stage weight loss behavior. The first decomposition stage around 230–350 °C was attributed to the decomposition of the sulfonic acid groups in the side chains, and the second decomposition stage around 450 °C was likely related to the degradation of main and side chains. The 5 wt% weight loss temperatures of the SNPAEK polymers were all higher than 230 °C, indicating that they had sufficient thermal stability for the PEM applications in medium temperature fuel cells.
IEC, water uptake and swelling ratio
Table 2 shows the IEC values of SNPAEK-xx membranes as determined through NMR method. IEC was a constant representing the amount of the exchangeable protons in the membrane. The calculated IEC values of SNPAEK-xx in acid-form membranes were in the range of 1.13 to 2.27 mequiv. g−1, which were close to the theoretical values derived from the molar ratio of 1,4-butane sultone to the hydroxyl content in HO-PAEK, indicating that the sulfonic acid groups were quantitatively grafted by sulfobutylation reaction.| Polymer membranes | IECa (mequiv g−1) | Water uptake (%) | Swelling ratio (%) | σ (S cm−1) | P (×10−7 cm2 s−1) | Selectivity (×104 S s cm−3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔT | ΔL | ||||||||||
| 25 °C | 80 °C | 25 °C | 80 °C | 25 °C | 80 °C | 25 °C | 80 °C | ||||
| a IEC was calculated by 1H NMR. | |||||||||||
| SNPAEK-0.65 | 1.13 | 9 | 14 | 2 | 3 | 2 | 3 | 0.010 | 0.027 | 1.25 | 8.00 |
| SNPAEK-1.00 | 1.60 | 20 | 31 | 2 | 7 | 3 | 10 | 0.035 | 0.092 | 4.42 | 7.92 |
| SNPAEK-1.30 | 1.90 | 30 | 52 | 4 | 10 | 5 | 13 | 0.061 | 0.145 | 7.32 | 8.33 |
| SNPAEK-1.47 | 2.14 | 34 | 79 | 5 | 16 | 7 | 17 | 0.076 | 0.181 | 9.28 | 8.19 |
| SNPAEK-1.60 | 2.27 | 37 | 101 | 6 | 17 | 7 | 20 | 0.094 | 0.191 | 10.22 | 9.20 |
| Nafion 117 | 0.92 | 18 | 28 | 5 | 14 | 11 | 17 | 0.075 | 0.149 | 23.80 | 3.15 |
The water uptake and swelling ratio of PEMs are significantly related to the membrane properties, such as proton conductivity, dimensional stability and mechanical properties. On one hand, the protons within PEMs could not be conducted unless they are hydrated by water;35 On the other hand, excessive water swelling would deteriorate the dimensional stability, thus resulting in the loss of mechanical properties. Therefore, the preparation of sulfonated polymers with appropriate water uptake is one of the critical requirements for their application as PEMs. The water uptake and swelling ratio of SNPAEK-xx membranes were evaluated by comparing the mass and dimensions of their hydrated state membranes with dry state membranes, respectively. Fig. 5 and Table 2 showed a clear trend of increasing water uptake with sulfobutyl content and IEC values at a given temperature. However, at lower sulfobutyl content, the water uptake of side-chain-type sulfonated polymers increased much slowly as the temperature increasing from 25 °C to 80 °C than those of main-chain-type sulfonated polymers. At a high sulfobutyl content, SNPAEK-1.60 membrane with a high IEC (2.27 mequiv. g−1) exhibited a sharply increase in water sorption. This indicated that the aggregation of the sulfonic acids at higher sulfobutyl content could induce the formation of the obvious phase separation morphology, which could lead to a rapid increase of water uptake.
As shown in Table 2, the swelling ratio also increased with the IEC and temperature increasing. But interestingly, all the SNPAEK-xx membranes showed a much lower swelling ratio, which were lower than those of main-chain-type sulfonated poly(arylene ether ketone)s.12 The side-chain-type sulfonated polymers locating the sulfonic acid groups on the flexible side chains were more effective in separating hydrophilic sulfonic acid groups from the hydrophobic polymer main chain. Thus, the molecular water would be restricted to hydrophilic domains and separated from hydrophobic domains, which suppressed the swelling behavior of the polymer membranes. Additionally, the rigidity naphthalene moieties in the polymer backbone remarkably increased the rigidity and hydrophobic of polymer chain, which can further restricted the water swelling at high IEC values. Although SNPAEK-1.60 has the highest IEC up to 2.27 mequiv. g−1, the swelling ratio is as low as 7% at 25 °C and 20% at 80 °C. This result indicates that the increasing introduction content of the naphthalene group and long alkylsulfonated side chains can effectively enhance the dimensional stability of the membranes.
Proton conductivity
The proton conductivity of SNPAEK-xx membranes at 100% RH was measured as a function of temperature and is displayed in Fig. 6. As the IEC values increased from 1.13 to 2.27 mequiv. g−1, the proton conductivity increased from 0.010 to 0.094 S cm−1 at 25 °C, and from 0.027 to 0.191 S cm−1 at 80 °C, which were suitable for practical applications as PEMs in fuel cells. Especially for SNPAEK-1.47 and SNPAEK-1.60, the proton conductivity was much higher than those of Nafion 117 (0.149 S cm−1 at 80 °C) and those of other side-chain-type SNPAEKs reported previously.3 It is well known that the proton conductivity of sulfonated aromatic polymers is mainly dependent on the IEC values. Especially for the randomly non-fluorinated sulfonated polymers, it required high IEC values to achieve high proton conductivity because of lower acidity of aliphatic sulfonic acid group.36 The morphology of the PEM is another crucial factor to determine the proton conductivity. For the side-chain-type SNPAEK-xx membranes, the sulfonic acid groups on the flexible aliphatic side chains would be beneficial to aggregate the ionic clusters, the highly rigid naphthalene groups on the polymer backbone could increased the free volume and hydrophobic of segments. Considering the hydrophilic sulfonic acid groups on the flexible side chains and the hydrophobic naphthalene moieties on the main chains, SNPAEK membranes are favor to form more continuous and larger transport channels at higher sulfobutyl content, and thus improving the proton conductivity.Methanol permeability and selectivity
Membranes for the practical application in DMFC must possess both high proton conductivity and low methanol permeability. Fig. 7 shows the 1H NMR spectra of methanol aqueous solution collected from the receptor compartment at a pre-set time in a diffusion cell, which was separated by a SNPAEK-xx membrane with different DS values. The methanol concentration for calculating the methanol permeability was measured by the deconvoluted peak area of the methyl groups of methanol (or hydroxyl proton of methanol) relative to the methylene groups of THF (internal standard) in the spectrum. The signal corresponding to the methyl groups (H1) and hydroxyl proton (H2) of methanol increased apparently as the IEC of SNPAEK-xx increased from 1.13 to 2.27 mequiv. g−1. As shown in Table 2, the calculated methanol permeability increased from 1.25 to 10.22 × 10−7 cm2 s−1, which was much lower than the value 2.38 × 10−6 cm2 s−1 of Nafion 117. As mentioned earlier, the transportation of methanol also required channels with good connectivity to form by the aggregation of hydrophilic clusters. Thus, the increasing trend of methanol permeability from SNPAEK-0.65 to SNPAEK-1.60 is easy to understand because of the increased water uptakes and IECs. The selectivity, which is defined as the ratio of proton conductivity to methanol permeability, is used to estimate the membrane potential performance in DMFC. As shown in Table 2, all the SNPAEK-xx membranes had the relatively higher selectivity than that of Nafion 117, indicating SNPAEK membranes have great potential for DMFC applications.Microstructure of the SNPAEK membranes
The morphology of SNPAEK-xx polymers was investigated by TEM. Fig. 8 shows the TEM micrographs corresponding to the morphologies of SNPAEK-1.30 and SNPAEK-1.60. In the TEM images, the dark areas corresponding to the ionic clusters stained by silver ions represent the hydrophilic domains and bright areas represent the domains formed by hydrophobic polymer backbones. The silver stained hydrophilic clusters are dispersed throughout the polymer matrix. With the increase of IEC, these silver clusters became more obvious and their density as well as average size of clusters increased. However, compared to Nafion membrane, the rigid fully aromatic main chains of SNPAEK hindered the formation of pronounced phase-separated morphology.37 It indicated that a higher IEC is still necessary to induce the flexible long alkyl side chains to aggregate into larger ionic domains and form better phase separation microstructure. SNPAEK-1.60 with the highest IEC values was observed to have more continuous and larger transport channels than that of SNPAEK-1.30 with lower IEC. The larger and connected hydrophilic domain was responsible to the efficient proton transportation and excellent proton conductivity. It was also contributed to the increase of water uptake and methanol permeability with IEC increasing.Conclusions
A series of novel side-chain-type fully aromatic naphthalene-based sulfonated poly(arylene ether ketone) polymers were successfully prepared from HO-PAEK derived from the original monomer. The IEC (1.13–2.27 mequiv. g−1) of SNPAEK-xx could be readily controlled by using different amount of 1,4-butane sultone and NaH. The polymers possessed high molecular weights, revealed by their high viscosities and formation of tough and flexible membranes. The SNPAEK-xx membranes showed anisotropic membrane swelling in water with lower swelling in thickness than in plane, which was helpful to improve the dimensional stability. From SNPAEK-1.30 to SNPAEK-1.60, they exhibited high proton conductivity, ranging from 0.145 S cm−1 to 0.191 S cm−1 at 80 °C. They also showed lower methanol permeability than that of Nafion 117. SNPAEK-1.30 and SNPAEK-1.60 showed the methanol permeability of 7.32 × 10−7 cm2 s−1 and 10.22 × 10−7 cm2 s−1, respectively. TEM images revealed that long alkylsulfonated side chains aggregated into hydrophilic clusters to form a continuous network at a sulfobutyl content of 1.60. The SNPAEK-xx membranes exhibited low methanol permeability, lower water swelling, and high proton conductivity and could be the promising materials for DMFC applications.Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant no. 21074044, 21374034, 21474036) and Science and Technology Development Plan of Jilin Province (Grant no. 20130522138JH).Notes and references
- C. H. Parka, C. H. Lee, M. D. Guiver and Y. M. Lee, Prog. Polym. Sci., 2011, 36, 1443–1498 CrossRef PubMed.
- L. Wu, Z. Zhang, J. Ran, D. Zhou, C. Li and T. W. Xu, Phys. Chem. Chem. Phys., 2013, 15, 4870–4887 RSC.
- K. Shao, J. Zhu, C. J. Zhao, X. F. Li, Z. M. Cui, Y. Zhang, H. T. Li, D. Xu, G. Zhang, T. Z. Fu, J. Wu, H. Na and W. Xing, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5772–5783 CrossRef CAS.
- K. Yoshimura and K. Iwasaki, Macromolecules, 2009, 42, 9302–9306 CrossRef CAS.
- S. J. Osborn, M. K. Hassan, G. M. Divoux, D. W. Rhoades, K. A. Mauritz and R. B. Moore, Macromolecules, 2007, 40, 3886–3890 CrossRef CAS.
- S. M. J. Zaidi, S. D. Mikhailenko, G. P. Robertson, M. D. Guiver and S. Kaliaguine, J. Membr. Sci., 2000, 173, 17–34 CrossRef CAS.
- K. Matsumoto, T. Higashihara and M. Ueda, Macromolecules, 2008, 41, 7560–7565 CrossRef CAS.
- M. Gil, X. L. Ji, X. F. Li, H. Na, J. E. Hampsey and Y. F. Lu, J. Membr. Sci., 2004, 234, 75–81 CrossRef CAS PubMed.
- K. Miyatake, Y. Chikashige and M. Watanabe, Macromolecules, 2003, 36, 9691–9693 CrossRef CAS.
- F. Wang, M. Hickner, Y. S. Kima, T. A. Zawodzinski and J. E. McGrath, J. Membr. Sci., 2002, 197, 231–242 CrossRef CAS.
- W. L. Harrison, F. Wang, J. B. Mecham, V. A. Bhanu, M. A. Hill, Y. S. Kim and J. E. Mcgrath, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 2264–2276 CrossRef CAS.
- P. X. Xing, G. P. Robertson, M. D. Guiver, S. D. Mikhailenko and S. Kaliaguine, Macromolecules, 2004, 37, 7960–7967 CrossRef CAS.
- K. B. Wiles, F. Wang and J. E. McGrath, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 2964–2976 CrossRef CAS.
- F. S. Nberger and J. Kerres, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 5237–5255 CrossRef.
- C. Genies, R. Mercier, B. Sillion, N. Cornet, G. Gebel and M. Pineric, Polymer, 2001, 42, 359–373 CrossRef CAS.
- X. X. Guo, J. H. Fang, T. Watari, K. Tanaka, H. Kita and K. Okamoto, Macromolecules, 2002, 35, 6707–6713 CrossRef CAS.
- K. Miyatake, T. Yasuda, M. Hirai, M. Nanasawa and M. Watanabe, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 157–163 CrossRef CAS.
- H. B. Zhang, J. H. Pang, D. Wang, A. Li, X. F. Li and Z. H. Jiang, J. Membr. Sci., 2005, 264, 56–64 CrossRef CAS PubMed.
- Y. Gao, G. P. Robertson, M. D. Guiver, S. D. Mikhailenko, X. Li and S. Kaliaguine, Macromolecules, 2005, 38, 3237–3245 CrossRef CAS.
- M. Y. Li, G. Zhang, S. Xu, C. J. Zhao, M. M. Han, L. Y. Zhang, H. Jiang, Z. G. Liu and H. Na, J. Power Sources, 2014, 255, 101–107 CrossRef CAS PubMed.
- M. B. Gieselman and J. R. Reynolds, Macromolecules, 1992, 25, 4832–4834 CrossRef CAS.
- Z. Y. Yang and R. G. Rajendran, Angew. Chem., 2005, 117, 570–573 CrossRef.
- E. M. W. Tsang, Z. B. Zhang, Z. Q. Shi, T. Soboleva and S. Holdcroft, J. Am. Chem. Soc., 2007, 129, 15106–15107 CrossRef CAS PubMed.
- K. Miyatake, T. Shimura, T. Mikamiac and M. Watanabe, Chem. Commun., 2009, 6403–6405 RSC.
- K. Nakabayashi, T. Higashihara and M. Ueda, Macromolecules, 2011, 44, 1603–1609 CrossRef CAS.
- K. Miyatake, T. Tombe, Y. Chikashige, H. Uchida and M. Watanabe, Angew. Chem., Int. Ed., 2007, 46, 6646–6649 CrossRef CAS PubMed.
- N. W. Li, C. Y. Wang, S. Y. Lee, C. H. Park, Y. M. Lee and M. D. Guiver, Angew. Chem., Int. Ed., 2011, 50, 9158–9161 CrossRef CAS PubMed.
- J. H. Pang, H. B. Zhang, X. F. Li, X. D. F. Ren and Z. H. Jiang, Macromol. Rapid Commun., 2007, 28, 2332–2338 CrossRef CAS.
- Y. Yin, J. H. Fang, T. Watari, K. Tanaka, H. Kita and K. Okamoto, J. Mater. Chem., 2004, 14, 1062–1070 RSC.
- N. Asano, M. Aoki, S. Suzuki, K. Miyatake, H. Uchida and M. Watanabe, J. Am. Chem. Soc., 2006, 128, 1762–1769 CrossRef CAS PubMed.
- L. E. Karlsson and P. Jannasch, J. Membr. Sci., 2004, 230, 61–70 CrossRef CAS PubMed.
- T. B. Norsten, M. D. Guiver, J. Murphy, T. Astill, T. Navessin, S. Holdcroft, B. L. Frankamp, V. M. Rotello and J. F. Ding, Adv. Funct. Mater., 2006, 16, 1814–1822 CrossRef CAS.
- J. F. W. McOmie, M. L. Watts and D. E. West, Tetrahedron, 1968, 24, 2289–2292 CrossRef CAS.
- L. Y. Zhang, D. Qi, G. Zhang, C. J. Zhao and H. Na, RSC Adv., 2014, 4, 51916–51925 RSC.
- M. F. H. Schuster, W. H. Meyer, M. Schuster and K. D. Kreuer, Chem. Mater., 2004, 16, 329–337 CrossRef CAS.
- K. D. Kreuer, S. J. Paddison, E. Spohr and M. Schuster, Chem. Rev., 2004, 104, 4637–4678 CrossRef CAS.
- Q. Zhang, B. J. Liu, W. Hu, W. Xu, Z. H. Jiang, W. Xing and M. D. Guiver, J. Membr. Sci., 2013, 428, 629–638 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12230e |
| This journal is © The Royal Society of Chemistry 2015 |