A novel sorbent for lanthanide adsorption based on tetraoctyldiglycolamide, modified carbon inverse opals
Abstract
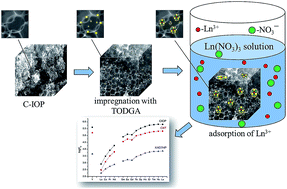
Carbon inverse opals (C-IOP) were noncovalently modified with tetraoctyldiglycolamide (TODGA). The effect of HNO3 concentration in the aqueous phase and that of the TODGA concentration in the sorbent phase on the adsorption of microquantities of Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu nitrates from HNO3 solutions by CIOP modified with TODGA was considered. The stoichiometry of the sorbed complexes has been determined by the slope analysis method. The efficiency of lanthanides(III) adsorption from moderate-concentration HNO3 solutions increases with increasing atomic number of the element. The process of lanthanides(III) adsorption is exothermic, spontaneous and followed a pseudo-second-order kinetic model.

 Please wait while we load your content...
Please wait while we load your content...