The upconversion and enhanced visible light photocatalytic activity of Er3+-doped tetragonal BiVO4
Wei Yang,
Guoqiang Tan*,
Huijun Ren,
Lili Zhang,
Chengcheng Zhao and
Ao Xia
Key Laboratory of Auxiliary Chemistry & Technology for Chemical Industry, Ministry of Education, Shaanxi University of Science and Technology, Xi'an, 710021, Shaanxi, China. E-mail: tan3114@163.com; Tel: +86 13759878391
First published on 22nd December 2014
Abstract
Er3+-doped BiVO4 with tetragonal structure is prepared by the microwave hydrothermal method. X-ray diffraction and Rietveld refinement demonstrate that the structure is transformed from the monoclinic (C2/c:c3) phase to the tetragonal (I41/amd:2) phase by doping with Er3+ ions. Er3+ doping also influences the morphology change of BiVO4 from irregular flake-like crystal to rod-like crystal, which leads to the increase of the surface areas from 3.25 to 11.96 m2 g−1. Compared with the monoclinic BiVO4, the upconversion of the Er3+-doped tetragonal BiVO4 occurs through the transitions from the 4I15/2 ground state to 4F7/2, 2H11/2, and 4F9/2 states, respectively. The photocatalytic experiment indicates that the tetragonal BiVO4 (8 at.%) with a larger specific surface area (9.88 m2 g−1) shows the best photocatalytic activity under visible light irradiation, which can efficiently improve the degradation rate of RhB up to 97.2% at 150 min.
1. Introduction
Semiconductor photocatalysts are widely used in the degradation of organic contaminants in wastewater.1–3 According to early reports, monoclinic BiVO4 (m-BiVO4) is the best visible-light-driven photocatalyst, while the photocatalytic activity under visible light irradiation of tetragonal BiVO4 (t-BiVO4) is negligible.4,5 Therefore monoclinic BiVO4 has been extensively studied as a promising photocatalyst for organic photocatalytic degradation due to its narrow band gap (2.4 eV).6,7 However, the rapid recombination rate of electron–hole pairs is always the main drawback for monoclinic BiVO4 to be applied. To solve this problem, different methods have been used to improve the photocatalytic activity of monoclinic BiVO4, such as co-catalyst loading, impurity doping, and the formation of a heterojunction.8,9 Recently, S. Usai and Sergio Obregón reported that Y3+ or Er3+ doping could induce the monoclinic–tetragonal heterostructure and improve the photocatalytic performance under sun-like excitation.10,11 On this basis, we obtained the tetragonal BiVO4 after doping Er3+ ions. This phenomenon is quite different from the previous reports. In this work, the Er3+-doped tetragonal BiVO4 system exhibits higher photocatalytic activity than that of monoclinic BiVO4 for the degradation of RhB under visible light irradiation.2. Experimental
All the reagents were of analytical grade and were used without any further purification. In a typical preparation process, 0.01 mol Bi(NO3)3·5H2O was dissolved in 25 mL deionized water and stirred for 20 min at room temperature. A quantity of 0.01 mol NH4VO3 was dissolved in 25 mL 100 °C deionized water and stirred for 20 min until the dissolution was complete. Then the NH4VO3 solution was added into the Bi(NO3)3·5H2O solution with magnetically stirring for 20 min, thus forming the jacinth slurry A. The pH of the slurry A was adjusted to 8.5 by adding concentrated NaOH (5 mol L−1). After stirred for several minutes, the appropriate contents of Er(NO3)3·6H2O were added to slurry A until the molar ratio of nEr to nBi was 0–12 at.%, generating precursors B. Then each precursor was transferred into the polytetrafluoroethylene (PTFE) autoclave (the filling density was 60%) and heated under microwave irradiation using a microwave hydrothermal reactor (Model MDS-8, Sineo, ShangHai). The specific program was as follow. Firstly, the microwave hydrothermal reaction power was set to 300 W and the temperature was from room temperature up to 100 °C for 6 min. Secondly, the microwave hydrothermal reaction temperature was from 100 °C to 150 °C for 6 min and from 150 °C to 200 °C for 40 min, and then it was allowed to cool to room temperature naturally. Finally, the obtained precipitates were washed three times with deionized water and absolute ethyl alcohol, and then dried at 80 °C for 12 h in air.The structural properties of the as-prepared samples were determined by an X-ray diffractometer (XRD, D/Max-2200, Rigaku, Japan) with Cu Kα radiation (λ = 0.15406 nm). The morphology was analyzed by using a scanning electron microscope (SEM, S-4800, Hitachi, Japan). The microstructures were investigated by a transmission electron microscopy (TEM) and a high resolution transmission electron microscopy (HRTEM, J EM-2100, Japan). The specific surface area was measured by a specific surface area analyzer (3H-2000BET-A, BJ, China). The adsorbate was N2 while the carrier gas was He. The UV-vis diffuse reflection spectra were recorded on a UV-vis spectrophotometer (Hitachi U-3900H, Japan), using BaSO4 as a reference. The upconversion fluorescence spectra were measured using YAG:Nd3+ (Quanta Ray Lab-170) pulse laser and Ti sapphire sapphire femtosecond laser (Mira-900) as excitation sources, and the output wavelength was 980 nm. The collection and detection of luminescence were carried out on a spectrometer (SP2750i) with a spectral resolution of 0.008 nm. All of the measurements were performed at room temperature. The Zeta potential was identified by Nano Particle and Zeta potential analyzer (Malvern Nano-ZS, UK).
In a process, a 500 W xenon lamp was used as a visible-light source. 0.05 g photocatalyst was dispersed in 50 mL RhB solution (5 mg L−1) under ultrasonic treatment. Prior to the irradiation, the solution was magnetically stirred in the dark for 30 min to achieve the adsorption–desorption equilibrium. The concentration of RhB was determined by recording the absorbance at 553 nm using a UV-vis spectrophotometer (Model SP-756p).
3. Results and discussion
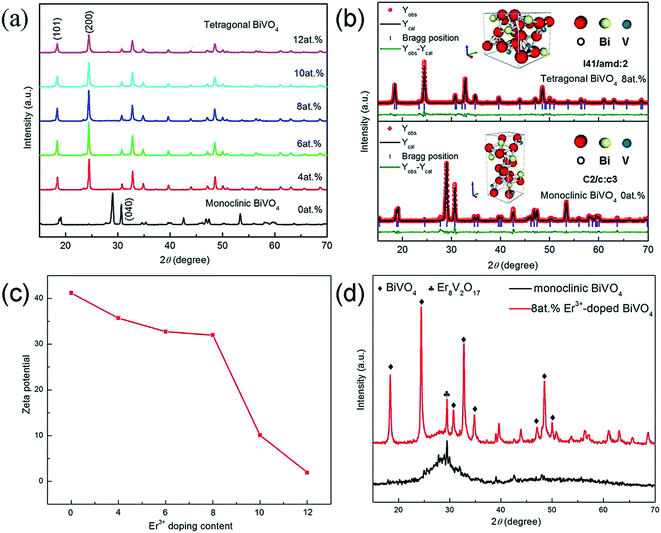
Fig. 1(a) shows the XRD patterns of pure BiVO4 and Er3+-doped BiVO4 photocatalysts. It is worth noting that the monoclinic BiVO4 (JCPDS no. 14-0688) was changed into the tetragonal BiVO4 (JCPDS no. 14-0133) when Er3+ is incorporated. The observed evolution of the crystalline structure of Er3+-doped BiVO4 is significantly different from that previously reported,11 that is merely the tetragonal phase is present when Er3+ content is above 4 at.%. In other words, Er3+-doped BiVO4 is considered to stabilize the tetragonal structure. In addition, the intensities of the (101) and (200) peaks of the tetragonal BiVO4 are reduced gradually so that the crystallinity of the composite is decreased.The Zeta potential of the precursor slurry is 41.20 with the Er3+-doped BiVO4 (0 at.%) in Fig. 1(c), suggesting the larger electrostatic repulsion, which is responsible for the shortage of BiVO4 crystal nuclei. The Bi3+ and VO3− will grow up into more stable thermodynamic monoclinic BiVO4 crystal grains according to certain spatial configurations and then irregular monoclinic BiVO4 in the microwave hydrothermal process.12 The Zeta potentials of Er3+-doped BiVO4 (4–12 at.%) are 35.71, 32.76, 32, 10.09, 1.94, respectively, suggesting the decreasing electrostatic repulsion, which can form a large number of BiVO4 crystal nuclei. The crystal nucleus will generate amorphous BiVO4 precipitate, which can be crystallized into tetragonal BiVO4 and Er8V2O17 in the drying oven at 80 °C (Fig. 1(d)). The tetragonal BiVO4 and Er8V2O17 are dissolved and crystallized into the tetragonal Er3+-doped BiVO4. The increase of Er3+ doping content give rise to the decrease of Er3+-doped BiVO4 crystal grains with recrystallization process (Table 1), resulting in the widened diffraction peak with the Er3+-doped BiVO4 (12 at.%).
| Samples | Crystal structure | Space group | Lattice parameters | Tetragonal crystallite size (nm) |
|---|---|---|---|---|
| Undoped | Monoclinic | C2/c:c3 | a = 5.1881 Å b = 5.0788 Å c = 11.6757 Å | |
| 4 at.% Er3+ | Tetragonal | I41/amd:2 | a = b = 7.2769 Å c = 6.4413 Å | 67.9 |
| 6 at.% Er3+ | Tetragonal | a = b = 7.2854 Å c = 6.4424 Å | 69.4 | |
| 8 at.% Er3+ | Tetragonal | a = b = 7.2894 Å c = 6.4464 Å | 69.1 | |
| 10 at.% Er3+ | Tetragonal | a = b = 7.2876 Å c = 6.4468 Å | 59.3 | |
| 12 at.% Er3+ | Tetragonal | a = b = 7.2852 Å c = 6.4465 Å | 49.4 |
To further analyze the crystal structure of the Er3+-doped BiVO4, the Rietveld refinement on the XRD patterns of pure BiVO4 and the Er3+-doped BiVO4 (8 at.%) is performed using the Maud program.13,14 In the light of the refinement results, the schematics of the BiVO4 crystal structure are shown in Fig. 1(b). The structural transition of Er3+-doped BiVO4 can be found, where the monoclinic BiVO4 and the Er3+-doped BiVO4 (8 at.%) having C2/c:c3 and I41/amd:2 space groups, respectively, can be observed.
The morphologies of the samples synthesized by microwave-hydrothermal process were characterized by a scanning electron microscopy (SEM), as shown in Fig. 2. When the content of Er3+ is 0 at.%, the crystal possesses irregular flake-like morphology with 100 nm thickness (Fig. 2(a)). The distribution of crystal grains is nonuniform and the gain surface is smooth, resulting in small specific surface area (3.25 m2 g−1). With the increase of doping Er3+ content (4–12 at.%), the flake-like crystal grains are dissolved gradually and recrystallized to the well-defined rod-like crystals of the tetragonal BiVO4 (Fig. 2(b)–(f)). This implies that the formation of crystals in nucleation–dissolution–recrystallization may be predominated by Er3+ ions. The increase of the specific surface area (from 7.42 m2 g−1 to 11.96 m2 g−1) can therefore be correlated with these changes of the morphology and smaller gain size. The changes of morphologies and the specific surface area attribute to the different Zeta potentials, resulting in the different growth of BiVO4 crystal. The tetragonal BiVO4 and Er8V2O17 crystals are dissolved gradually and recrystallized to form the well-defined rod-like crystals. These results are in accordance with the XRD patterns.
 | ||
| Fig. 2 SEM images of Er3+-doped BiVO4 (a) 0 at.% (b) 4 at.% (c) 6 at.% (d) 8 at.% (e) 10 at.% (f) 12 at.%. (g) Specific surface areas of the monoclinic BiVO4 and Er3+-doped BiVO4. | ||
It can also be seen in the TEM images that the irregular flake-like crystal is the monoclinic BiVO4 (0 at.%) with 100 nm thickness (Fig. 3(a)) and the rod-like crystal is Er3+-doped BiVO4 (8 at.%) with the length of 300 nm (Fig. 3(c)). From the insets in Fig. 3(b) and (d), the SEAD diffraction patterns are the bright spots of electron diffraction, which indicates that both monoclinic and tetragonal BiVO4 are crystalline. The HRTEM images obtained from the (b and d) areas marked with rectangles are shown in Fig. 3(b) and (d). An interval of 0.2910 nm between the uniform fringes is in good agreement with the (040) lattice plane of monoclinic BiVO4. Meanwhile, the spaces of 0.4810 nm and 0.3626 nm of the clear lattice fringes with corresponding to (101) and (200) planes of tetragonal BiVO4 imply the high crystallinity. These results are consistent with the initial XRD analyses.
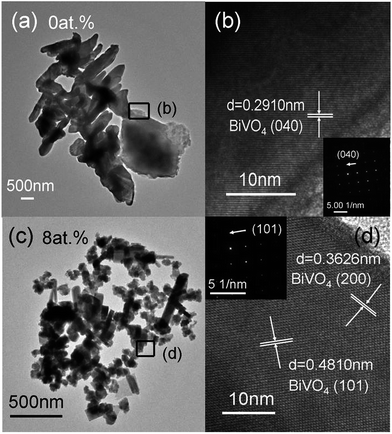 | ||
| Fig. 3 (a and b) TEM images and HRTEM of monoclinic BiVO4 (0 at.%); (c and d) TEM images and HRTEM of Er3+-doped BiVO4 (8 at.%) (the insets in (b) and (d) show SEAD patterns). | ||
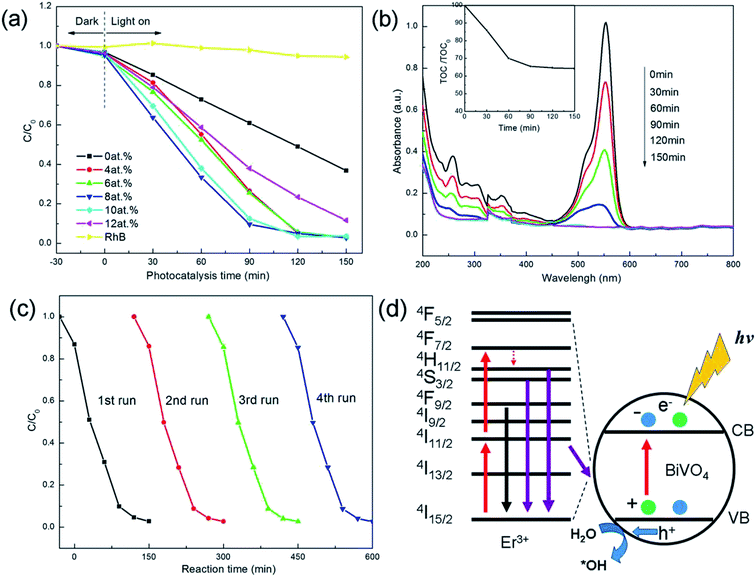
The optical properties of monoclinic BiVO4 and Er3+-doped tetragonal BiVO4 are measured by using UV-vis diffuse reflection spectrum in the wavelength range of 200–800 nm. From the diffuse reflectance spectra (Fig. 4(a)), two clear absorption edges can be noticed both in the UV and visible light regions, which corresponds to the band gap energy of 2.4 eV and 2.9 eV of the monoclinic and tetragonal phases, respectively.15 Compared with the monoclinic BiVO4, three new absorption bands at ca. 490, 524 and 655 nm of Er3+-doped BiVO4 can be observed, which can be assigned to the Er3+ transitions from the 4I15/2 ground state to 4F7/2, 2H11/2, and 4F9/2 states, respectively.16
 | ||
| Fig. 4 (a) UV-vis diffuse reflection absorption spectra of Er3+-doped BiVO4. (b) The upconversion fluorescence spectra of Er3+-doped tetragonal BiVO4 (8 at.%) upon 980 nm laser excitation. | ||
In order to understand the luminescent property of Er3+ doping in the catalysts, we have performed the upconversion fluorescence spectra of Er3+-doped tetragonal BiVO4 (8 at.%) upon 980 nm laser excitation (Fig. 4(b)). In the spectrum, there are two emission parts: the green emission from 490 nm to 570 nm is corresponding to the transitions from 2H11/2/4F7/2 to 4I15/2 when Er3+ ions are doped and the red one in a range 640–690 nm is assigned to the transition of 4F9/2–4I15/2 when Er3+ ions are doped.17 The result indicates the existence of the upconversion process in the Er3+-doped tetragonal BiVO4 photocatalyst. Generally, the Er3+-doped upconversion process can increase the number of incoming photons with adequate energy. Then the photons can be absorbed by the photocatalyst, which will generate more photogenerated electron–hole pairs.18 Therefore, the photocatalytic activities of Er3+-doped tetragonal BiVO4 with up-conversion effect are expected to be efficiently enhanced.
The photocatalytic activities of the Er3+-doped BiVO4 are evaluated by measuring the degradation of RhB aqueous solution under visible light irradiation shown in Fig. 5(a). For the monoclinic BiVO4, the photodegradation of RhB is 63.13% after 150 min irradiation. By introducing the up-conversion Er3+ and optimizing the contents (4–12 at.%), the photodegradation rates of RhB have promptly reached to 96.80%, 96.98%, 97.2%, 96.38% and 88.35% respectively, which exhibits much higher photocatalytic activity than that of the monoclinic BiVO4. When Er3+ is increased to 10 at.%, the photocatalytic activity begins to be decreased because the excessive Er3+ ions may cover the active sites on BiVO4 surface and also may act as the recombination centers of photogenerated electrons and holes, resulting in the decrease of photocatalytic activity.19 Therefore, the optimum content of doping Er3+ is 8 at.%.
The temporal evolution of spectral changes during the photodegradation process of RhB for Er3+-doped BiVO4 (8 at.%) is displayed in Fig. 5(b). From the spectra, it can be seen that the absorbance of RhB at the maximum absorption wavelength is gradually decreased, which realizes the de-ethylation. Under visible illumination, the intense bright cherry-red color of the starting solution gradually disappears with increasing exposure time. The de-ethylation of the N,N,N,N-tetraethylated rhodamine molecules appears in the wavelength position of its major absorption band moving toward the blue region, and it is as follows: λmax, RhB, 554 nm; N,N,N-triethylated rhodamine, 539 nm; N,N-diethylated rhodamine, 522 nm; and N-ethylated rhodamine, 510 nm; and rhodamine, 498 nm. RhB has completely been changed into rhodamine.20 Then through the destruction of their conjugate structure, the further degradation is achieved. After a series of complicated oxidation reaction, the RhB is decomposed to smaller organics and minerals, which can be proved by the results of the TOC measurement.21 The inset in Fig. 5(b) displays that the ability of Er3+-doped tetragonal BiVO4 (8 at.%) to mineralize RhB is evaluated by monitoring changes of organic carbon in TOC. As can be seen, TOC diminishes gradually and ca. 36% TOC removal is obtained after 150 min irradiation, implying that RhB degradation is accompanied by partial mineralization.
The recycle experiments are performed to evaluate the photocatalytic repeatability and stability of Er3+-doped tetragonal BiVO4 (8 at.%) photocatalyst under visible light irradiation.22 Fig. 5(c) shows the results from the four successive runs of the photodegradation of RhB under the same experimental conditions. It is clear to see that no significant photoactivity loss is observed after four times of successive recycles. The results indicate that Er3+-doped tetragonal BiVO4 (8 at.%) photocatalyst is stable during the photodegradation of RhB.
Fig. 5(d) shows the mechanism of the upconversion of Er3+-doped tetragonal BiVO4. For monoclinic BiVO4, the absorbable light is limited to 519 nm (Fig. 4(a)), while the proper doping content of Er3+ in the Er3+doped BiVO4 with can effectively be converted from the long wavelength light to the short wavelength light so as to generate the excited electrons with higher energy and sequentially to capture low energy photons as shown in Fig. 5(d). When these excited electrons are on the ground state by means of irradiation or energy transformation, the emitted light or the transformed energy will make the tetragonal BiVO4 yield more photoinduced electron–hole pairs so as to enhance the photocatalytic activities. Then the highly oxidative holes accumulated in the BiVO4 can directly decompose the organic pollutants or react with H2O molecules to produce ˙OH,23,24 which indicates that the upconversion process can enhance the utilization of efficient photons of Er3+-doped tetragonal BiVO4 photocatalysts. On the basis, the upconversion plays a significant role in the improvement of the photocatalytic activities in the Er3+-doped tetragonal BiVO4 system.
4. Conclusions
The tetragonal BiVO4 with different Er3+-doped contents has been successfully synthesized by microwave hydrothermal method. The morphology of Er3+-doped BiVO4 has been changed from irregular flake-like to rod-like crystals with the structural transition from monoclinic to tetragonal BiVO4. Compared with the monoclinic BiVO4, the upconversion of the Er3+-doped tetragonal BiVO4 occurs through the transitions from the 4I15/2 ground state to 4F7/2, 2H11/2, and 4F9/2 states, which makes the tetragonal BiVO4 generate more photoinduced electron–hole pairs so as to enhance the photocatalytic activities. Furthermore, the Er3+-doped tetragonal BiVO4 exhibits significantly higher photocatalytic activity than that of the monoclinic BiVO4 for the degradation of RhB under visible light irradiation.Acknowledgements
This work is supported by the Project of the National Natural Science Foundation of China (Grant no. 51172135); the Academic Leaders Funding Scheme of Shaanxi University of Science & Technology (2013XSD06); Doctorate Scientific Research Initial Fund Program of Shaanxi University of Science & Technology(BJ4-13); the Graduate Innovation Fund of Shaanxi University of Science & Technology (SUST-A04).References
- S. Y. Dong, Y. R. Cui, Y. F. Wang, Y. K. Li, L. M. Hu, J. Y. Sun and J. H. Sun, Chem. Eng. J., 2014, 249, 102–110 CrossRef CAS PubMed.
- O. Y. Ke, S. Xie and X. O. Ma, Ceram. Int., 2013, 39, 7531–7536 CrossRef PubMed.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- H. Y. Jiang, H. X. Dai, X. Meng, K. M. Ji, L. Zhang and J. G. Deng, Appl. Catal., B, 2011, 105, 326–334 CrossRef CAS PubMed.
- C. Karunakaran, S. Kalaivani, P. Vinayagamoorthy and S. Dash, Mater. Sci. Semicond. Process., 2014, 21, 122–131 CrossRef CAS PubMed.
- X. M. Qi, X. Y. Zhu, J. Wu, Q. Wu, X. Li and M. L. Gu, Mater. Res. Bull., 2014, 59, 435–441 CrossRef CAS PubMed.
- S. Y. Dong, J. L. Feng, Y. K. Li, L. M. Hu, Me. L. Liu, Y. F. Wang, Y. Q. Pi, J. Y. Sun and J. H. Sun, Appl. Catal., B, 2014, 152, 413–424 CrossRef PubMed.
- P. Ju, P. Wang, B. Li, H. Fan, S. Y. Ai, D. Zhang and Y. Wang, Chem. Eng. J., 2014, 236, 430–437 CrossRef CAS PubMed.
- Y. X. Ji, J. F. Cao, L. Q. Jiang, Y. H. Zhang and Z. G. Yi, J. Alloys Compd., 2014, 590, 9–14 CrossRef CAS PubMed.
- S. Usai, S. Obregón, A. I. Becerro and G. Colón, J. Phys. Chem. C, 2013, 117, 24479–24484 CAS.
- S. Obregón and G. Colón, Appl. Catal., B, 2014, 158, 242–249 CrossRef PubMed.
- L. Zhou, W. Z. Wang and L. S. Zhang, J. Phys. Chem. C, 2007, 111, 13659–13664 CAS.
- C. Karunakaran, S. Kalaivani, P. Vinayagamoorthy and S. Dash, Mater. Sci. Eng., B, 2014, 187, 53–60 CrossRef CAS PubMed.
- R. Venkatesan, S. Velumani and A. Kassiba, Mater. Chem. Phys., 2012, 135, 842–848 CrossRef CAS PubMed.
- S. M. Thalluri, C. M. Suarez, S. Hernández, S. Bensaid, G. Saracco and N. Russo, Chem. Eng. J., 2014, 245, 124–132 CrossRef CAS PubMed.
- S. Obregón and G. Colón, RSC Adv., 2014, 4, 6920–6926 RSC.
- Q. Lu, Q. H. Yang, Y. Yuan, C. Jiang and Y. G. Wang, Ceram. Int., 2014, 40, 7367–7372 CrossRef CAS PubMed.
- S. Obregón and G. Colón, Appl. Catal., B, 2013, 140, 299–305 CrossRef PubMed.
- H. Liu, M. Luo, J. C. Hu, T. F. Zhou, R. Chen and J. L. Li, Appl. Catal., B, 2013, 140, 141–150 CrossRef PubMed.
- U. M. García-Pérez, S. Sepúlveda-Guzmán and A. Martínez-de la Cruz, Solid State Sci., 2012, 14, 293–298 CrossRef PubMed.
- H. Tang, D. Zhang, G. G. Tang, X. R. Ji, C. S. Li, X. H. Yan and Q. Wu, J. Alloys Compd., 2014, 591, 52–57 CrossRef CAS PubMed.
- J. Zhang, H. Cui, B. Wang, C. Li, J. P. Zhai and Q. Li, Chem. Eng. J., 2013, 223, 737–746 CrossRef CAS PubMed.
- H. Zheng, B. J. Chen, H. Q. Yu, J. S. Zhang, J. S. Sun, X. P. Li, M. Sun, B. N. Tian, H. Zhong, S. B. Fu, R. N. Hua and H. P. Xia, RSC Adv., 2014, 4, 47556–47563 RSC.
- Y. Guan, Z. H. Wei, Y. L. Huang, R. Maalej and H. J. Seo, Ceram. Int., 2013, 39, 7023–7027 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |