Secondary amine based ionic liquid: an efficient catalyst for solvent free one pot synthesis of xanthenes and benzoxanthenes†
Abstract
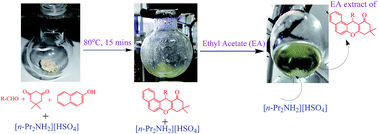
Ionic liquids (IL) prepared from dialkylamines and concentrated sulphuric acid were used in an operationally simple, cost effective, efficient and environmentally benign synthesis of xanthenes and benzoxanthenes. Three ILs were screened for their efficiency as promoter of the synthesis. Results indicate di-n-propylammoniumhydrogensulphate to be the most efficient IL. Product recovery is simple and the IL could be recovered for reuse.

 Please wait while we load your content...
Please wait while we load your content...