Lipid/detergent mixed micelles as a tool for transferring antioxidant power from hydrophobic natural extracts into bio-deliverable liposome carriers: the case of lycopene rich oleoresins†
Abstract
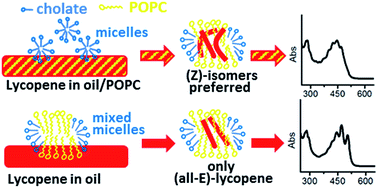
This work demonstrates that lipid-detergent mixed micelles can be employed successfully in order to achieve and modulate the transfer of bio-active hydrophobic compounds into lipid carriers by means of a simple and bio-safe procedure. In our specific investigation, liposome preparations incorporating mixtures of natural carotenoids with high lycopene content were developed and characterized, aiming to obtain formulations of potential nutraceutical and pharmaceutical interest. The starting material was a solvent-free high-quality lycopene rich oleoresin (LRO) obtained by extracting a freeze-dried tomato matrix with supercritical carbon dioxide (SC-CO2). Mixed micelles containing 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and cholate were loaded with LRO antioxidants by means of two slightly different procedures, which surprisingly resulted in significant differences in both quality and quantity of incorporated carotenoids. In particular, the selective incorporation of (all-E)-lycopene was achieved by extracting the oleoresin with a pre-formed cholate/POPC micelle suspension whilst (Z)-isomers were preferentially integrated when treating a POPC/LRO mixed film with cholate. The micelle to vesicle transition (MVT) method was employed in order to produce vesicles of well-defined lamellarity and size. Visible and infrared (IR) spectroscopy as well as Dynamic Light Scattering (DLS) and Transmission Electron Microscopy (TEM) measurements allowed the extensive characterization of LRO-loaded micelles and liposomes. The antioxidant potential of preparations was assessed by measuring the radical scavenging activity towards the coloured radical cation of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonate) (ABTS). Important information about the reliability of different approaches for antioxidant capacity evaluation of micelle and liposome preparations was gained and the successful incorporation of LRO antioxidant power in a bio-deliverable water-dispersed form was demonstrated.

 Please wait while we load your content...
Please wait while we load your content...