New 1,4-anthracenedione derivatives with fused heterocyclic rings: synthesis and biological evaluation†
Abstract
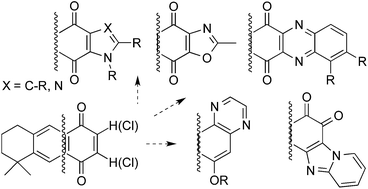
Several terpenylquinones derived from 1,4-anthracenedione (1,4-anthracenequinone, AQ) have been prepared by addition or substitution nucleophilic reactions and further transformed into extended polycyclic systems, which mainly kept the 1,4-quinone moiety fused to different nitrogen-heterocyclic rings (pyrrole, imidazole, pyrazine or quinoxaline) into the structure. The compounds synthesized were evaluated for their antineoplastic, antifungal and antiviral activities. GI50 antineoplastic values remained under μM levels for AQs, while the heterocyclic derivatives showed antifungal MIC values in the low μg mL−1 range against yeasts and filamentous fungi. Only few compounds displayed a discrete non-selective antiherpetic activity in the μg mL−1 range.

 Please wait while we load your content...
Please wait while we load your content...