An efficient thermoelectric material: preparation of reduced graphene oxide/polyaniline hybrid composites by cryogenic grinding
Weijie Wanga,
Qihao Zhanga,
Jianlin Lib,
Xia Liua,
Lianjun Wang*a,
Juanjuan Zhua,
Wei Luoa and
Wan Jiang*ac
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai 201620, People's Republic of China. E-mail: wanglj@dhu.edu.cn
bKey Laboratory of Ministry of Education for Advanced Materials in Tropical Island Resources, School of Materials and Chemical Engineering, Hainan University, 58 Renming Ave, Haikou 570228, China
cSchool of Material Science and Engineering, Jingdezhen Ceramic Institute, Jingdezhen 333000, China
First published on 18th December 2014
Abstract
An alternative and facile strategy to fabricate conducting reduced graphene oxide/polyaniline (rGO/PANI) hybrid composites with highly enhanced thermoelectric properties is introduced. rGO and PANI were homogeneously mixed by cryogenic grinding and then consolidated via spark plasma sintering. X-ray diffraction, X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy and transmission electron microscopy were employed to evaluate the phase structure and microstructure of the as-prepared composites. The results show that the CG technique could not only effectively refine the grain size of PANI, but also could induce more dislocations. The refined PANI particles are homogeneously dispersed and orderly arranged on the rGO templates as a result of the strong π–π conjugated interactions between PANI and rGO. The thermoelectric properties of the PANI samples containing different rGO content were systematically investigated. Compared with pure bulk PANI, rGO/PANI hybrid composites exhibit a distinct enhancement in the thermoelectric performance. Both the Seebeck coefficient and the electric conductivity were found to increase remarkably, resulting from the increased carrier mobility. The maximum Seebeck coefficient and electric conductivity of the rGO/PANI hybrid composites amazingly reached 15.934 μV K−1 and 1858.775 S m−1, respectively, and the maximum ZT was up to 4.23 × 10−4.
Introduction
Searching and developing new, clean, effective and reproducible energy has become one of the most critical issues. Thermoelectric (TE) materials could achieve the mutual conversion between thermal energy and electric energy, which has received renewed attention due to their great potential for applications in Peltier coolers and thermoelectric power generators.1,2 As is known, the performance of thermoelectric material is determined by its dimensionless figure of merit ZT (ZT = S2σTκ−1, where S, σ, κ and T are the Seebeck coefficient, electrical resistivity, thermal conductivity and absolute temperature, respectively). It is obvious that a high ZT should possess high σ, large S and low κ. Over the past half century, the question of how to enhance ZT over 1 for practical use has become a highly important topic in research.3 Until now, the performance research of TE materials have mainly focused on inorganic semiconductors, such as PbTe, Bi2Te3, CoSb3, SnSe and theirs alloys or composites.4–8 Compared with inorganic thermoelectric materials, conducting polymer thermoelectric materials (such as poly(3,4-ethylenedioxythiophene),9 polyaniline,10 polypyrrole11) having intrinsically low thermal conductivity, low toxicity, mechanical flexibility and inexpensive possibility have been widely considered as potential candidates for TE materials.12 Particularly, due to the low cost, structural diversification, unique doping/dedoping progress, low thermal conductivity, and the easy to synthesize nature, PANI is regarded as one of the most potential effective and suitable TE materials among conducting polymers.13 However, the power factor (S2σ) for PANI thermoelectric materials is in the range of 10−6–10−10 W m−1 K−2, which leads to a serious lag in its large-scale application.14 In the last decade, carbon materials, such as graphite oxide, CNTs and graphene have been introduced into the PANI matrix to enhance its thermoelectric properties.15–18Graphene, as a new form of carbon, has attracted considerable interest because of its intriguing two-dimensional sheet of sp2-bonded, 3 single-atom-thick graphene. In addition to the thinness, mechanical strength and material flexibility, graphene, a single layer of carbon atoms arranged in a hexagonal honeycomb lattice, has some outstanding physical properties, such as high carrier mobility (in excess of 105 cm2 V−1 s−1) and high thermal conductivity (up to 5000 W m−1 K−1).19,20 Because of these excellent performance characteristics, a much wider application of these materials and composite materials, especially for graphene composites with polyaniline, is possible, such as in resonators, catalyst supports, electronic devices, supercapacitors, batteries and solar cells.21–27 The strong π–π interaction between the graphene structure and aromatic rings of polyaniline would facilitate electron delocalization and improve the electrical conductivity of the composites.28 Nevertheless, several of these applications are still not feasible because the large-scale production of pure grapheme sheets remains challenging. The chemical reduction of graphite oxide (GO) is one of the established procedures to make graphene in large volume.29 Many primary products with high conductivity but poor solubility have been made by chemical reduction. However, the lower content of oxygenic groups on the surface of rGO often results in severe aggregation and agglomeration. To improve the dispersibility of rGO in composites, some progress has been made by using surfactants.30 For example, Zhang et al. prepared chemically modified graphene and polyaniline fiber composites by in situ polymerization of aniline monomer in the presence of graphene oxide under acidic conditions and then used hydrazine as a reducing agent.31 Kumar et al. prepared graphene oxide/polyaniline with a mild oxidant by in situ polymerization.32 Graphene ultrasonic was treated with a mixture of aniline monomer and ammonium persulfate to form PANI on its surface by Al-Mashat and his colleagues.33 However, all the above methods are complex and time-consuming. Thus, it is necessary to find a simple, low-cost and environmentally friendly way to prepare rGO/PANI composites.
In this work, we report a novel and green method for fabricating nanostructured rGO/PANI composites. As-synthesized rGO/PANI composite powder was prepared using cryogenic grinding (CG) without any dispersant.34 After the CG process, the grain size of PANI had been refined, and rGO sheets are also uniformly dispersed in the composites. PANI is well-alignedly dispersed on the rGO template because of the strong π–π conjugated interactions between PANI and rGO. Finally, the composite's powers are consolidated via spark plasma sintering (SPS). With the rGO template, carrier transports are improved between the interchain and intrachain of PANI following the variable range hopping.35 An ordered chain arrangement reduces the barrier to both interchain hopping and intrachain hopping and enhances the carrier mobility.36 Moreover, it has been found that rGO has extraordinary electrical conductivity, which produces an effective conductive medium for carrier transmission in rGO/PANI composites. Therefore, the electrical conductivity and power factor of composites are found to increase with the addition of rGO.
Materials synthesis
1. Preparation of PANI
Aniline (99.9%, monomer) and ammonium peroxydisulfate (APS, initiator) were obtained from Sinopharm Chemical Reagent Co. Ltd. Aniline could not be used until it is distill-purified. Corresponding solutions were prepared using deionized water during the synthesis process.In a typical synthesis of PANI, solution A is as follows: 10 mL of aniline was diluted with 200 mL of 1 M HCl. Then, 20 g of (NH4)S2O8 dissolved in 200 mL of 1 M HCl was slowly added into solution A under stirring to form a brown slurry. The polymerization reaction was carried out for 5 h at 0 °C. The mixture was filtered, washed three times with deionized water, and finally dried at 60 °C in a vacuum oven.
2. Preparation of reduced graphene oxide
Synthesis of GO: GO was prepared according to the modified Hummers method, reported elsewhere.37 1 g of purified natural graphite powder was added to a flask and filled with concentrated cold H2SO4 (25 mL) at 0 °C, followed by addition of KMnO4 (3.5 g) gradually at 0 °C (ice bath). After slowly raising the temperature to 40 °C, the mixture was stirred for 2 h. Water (200 mL) was added at least three times to the mixture, and then H2O2 (30 wt%, 5 mL) was added at least three times to react with excess KMnO4. The as-prepared GO precipitated quickly after 3 hours because of the strongly acidic environment. The precipitate mixture was washed 5 times with HCl solution (1 M, 1 L). Then, water (1 L) was added to the mixture. Finally, the colloid was centrifuged at 8000 rpm for 60 min to remove graphite and large GO flakes. The obtained GO colloid was used to prepare all composite samples.The reduced graphene oxide was prepared according to the method of Gao et al.38 The GO colloid was dispersed in water to give a 1.0 g L−1 colloidal solution. A 5 wt% sodium carbonate solution was used to adjust the PH of the solution to 9–10. 1600 mg sodium borohydride was directly added into 200 mL GO dispersion under magnetic stirring for 30 min, and the mixture was kept at 90 °C for 1 h with constant stirring. Then, the product was filtered and washed several times with plenty of water. This partially reduced GO was redispersed in concentrated sulfuric acid and heated to 120 °C (oil bath) with stirring for 12 h. After cooling, large volume of deionized water was added to the dispersion. The final product was thoroughly rinsed with water. The product powder was further annealed at 1100 °C under gas flow of Ar with 15 vol% H2 for 15 min.
3. Preparation of rGO/PANI composites
For the rGO/PANI composites, the samples were fabricated through cryogenic grinding by a CG machine (SPEX SamplePrep 6770 Freezer/Mill, TECH-Knowledge International Co., California, USA). The sample, plugs and impactor were embedded in a granding vial; the vial was then precooled in liquid nitrogen to make the sample brittle. 1 g polyaniline was ground at −195.6 °C in the liquid nitrogen for 40 min, then the sample containing polyaniline and different mass fractions (0, 5%, 10%, 20%, 30%) of rGO was ground at −195.6 °C for 40 min. Finally, the as-milled composite powders were consolidated at 100 °C for 10 min by a spark plasma sintering (SPS, Dr Sinter 725; Sumitomo Coal Mining Co., Tokyo, Japan).Characterization
Powder X-ray diffraction (PXRD) measurement was performed using a diffractometer (D/Max-2550PC) with Cu Kα (λ = 0.15406 nm) radiation. The structures of composite samples were measured by a Nicolet 8700 FTIR spectrometer. The spectra were collected by averaging 32 scans, ranging from 500 to 4000 cm−1. The morphology of rGO was observed by transmission electron microscopy (TEM, Model 2100F, Japan) with a selected area electron diffraction (SAED). Raman spectra were collected using an Avalon Instruments Raman Station with a 532 nm He–Ne laser. X-ray photoelectron spectroscopy (XPS) was performed on a PHI Quantera SXM Scanning X-ray Microprobe with an Al cathode (hν = 1486.6 eV) as the X-ray source set at 100 W and a pass energy of 26.00 eV. For the thermoelectric properties, the resistivity and Seebeck coefficient were investigated by Seebeck coefficient/electric conductivity measuring system (ZEM-3). The thermal diffusivity was investigated by a laser-flash method on a disk using a commercial system (LFA427; Netzsch Instruments, Selb, Germany). The Hall coefficient RH measurement of the sample was carried out on a PPMS system (Quantum Design INC., USA) with a magnetic field of 2 T and an electrical current of 30 mA.Morphology characterization
As shown in Fig. 1, significant structural changes occur during the chemical processing from GO to rGO. PANI and the formation of PANI/rGO composites are reflected in the Raman spectra. As expected, the Raman scattering of the as-prepared GO and rGO display two prominent Raman-active peaks at 1330 and 1590 cm−1, which correspond to the well-documented D mode of a sp2-hybridized carbon and the G mode related to the vibration of a sp3, hybridized carbon, respectively.32,39–41 It is worth noting that the intensity ratio of D and G bands has been widely used as an indicator of the amount of disorders. Moreover, we can observe that GO has a lower ID/IG compared to rGO, which indicates that GO have been successfully reduced. For the PANI sample, the C–H bending of quinoid ring at 1173 cm−1, the C–H bending of the benzenoid ring at 1255 cm−1, C–N+ stretching at 1344 cm−1, and C–C stretching of the benzene ring at 1499 cm−1 are obviously observed. For PANI/rGO composites, the reduced intensities of two peaks (D band and G band) in the spectra of PANI/rGO at 1330 and 1590 cm−1 are probably due to the strong interactions between PANI and rGO.42 Therefore, the Raman spectra demonstrate that PANI has dispersed on the rGO sheets.The FTIR spectra for the rGO, pure PANI and PANI/rGO composites with the increasing of rGO content from 5% to 30% are shown in Fig. 2. As is commonly observed for PANI, the quinoid band is less intense than the benzenoid band. The peaks at 804 cm−1, 1130 cm−1, 1397 cm−1, 1638 cm−1 and 3435 cm−1 can be assigned to the C–H out-of-plane bending vibration, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching (–N
N stretching (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) quinoid
quinoid![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) vibration, C–N stretching vibration in aromatic, C
N–) vibration, C–N stretching vibration in aromatic, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and N–H stretching mode, respectively.43,44 rGO shows the presence of strong bands at around 1636 and 1109 cm−1, characteristic of C
O stretching vibration, and N–H stretching mode, respectively.43,44 rGO shows the presence of strong bands at around 1636 and 1109 cm−1, characteristic of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–C, respectively, associated with the stretching modes of the ester linkage.45 Several new peaks attributed to PANI appear in the spectrum of PANI/rGO. Notably, many low-intensity peaks ranging from 580 to 780 cm−1 can be assigned to the vibrations of the C–H bonds in the benzene rings. The band at about 800 cm−1 could be attributed to the C–H out-of-plane bending vibrations. In addition, a stretching band assigned to C–N also appears at 1300 cm−1.46 The appearance of the quinonoid and benzenoid ring vibrations (C
C and C–C, respectively, associated with the stretching modes of the ester linkage.45 Several new peaks attributed to PANI appear in the spectrum of PANI/rGO. Notably, many low-intensity peaks ranging from 580 to 780 cm−1 can be assigned to the vibrations of the C–H bonds in the benzene rings. The band at about 800 cm−1 could be attributed to the C–H out-of-plane bending vibrations. In addition, a stretching band assigned to C–N also appears at 1300 cm−1.46 The appearance of the quinonoid and benzenoid ring vibrations (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching deformations) at about 1564 and 1461 cm−1, respectively, clearly indicates the presence and formation of PANI on the graphene surfaces. As is commonly observed, the quinonoid band at 1564 cm−1 is less intense than the benzenoid band at 1461 cm−1.47 The characteristic band attributable to the N–Q–N–Q stretch of the quinonoid ring is also found at around 1144 cm−1, which clearly supports our hypothesis that PANI has been covalently dispersed onto the surface of the rGO sheets because of the strong π–π conjugation interaction.48
C stretching deformations) at about 1564 and 1461 cm−1, respectively, clearly indicates the presence and formation of PANI on the graphene surfaces. As is commonly observed, the quinonoid band at 1564 cm−1 is less intense than the benzenoid band at 1461 cm−1.47 The characteristic band attributable to the N–Q–N–Q stretch of the quinonoid ring is also found at around 1144 cm−1, which clearly supports our hypothesis that PANI has been covalently dispersed onto the surface of the rGO sheets because of the strong π–π conjugation interaction.48
 | ||
| Fig. 2 FTIR spectra of rGO, CGed–PANI, PANI, CGed–PANI/(5 wt%)rGO, CGed–PANI/(10 wt%)rGO, CGed–PANI/(20 wt%)rGO, and CGed–PANI/(30 wt%)rGO. | ||
Fig. 3 shows the X-ray diffraction patterns for rGO, PANI, CGed–PANI and CGed–PANI/rGO composites. The XRD peaks from PANI are observed at 2θ = 14.92°, 20.74° and 25.28°, corresponding to the (011), (020) and (200) reflections of polyaniline in its emeraldine salt form, respectively.49 From Fig. 3b and c, it can be seen that the peaks of polyaniline are substituted by a broad peak, indicating that the grain size of PANI has decreased sharply and many more dislocations may occur during the cryogenic grinding process. Meanwhile, the XRD peaks from rGO are observed at 2θ = 25.4°, indicating the successful reduction of GO.50 No obvious PANI peaks, but obvious rGO, are observed in the XRD pattern for the PANI/rGO composites. This could possibly be attributed to two reasons. One is that the size of PANI/rGO decreases sharply and the partial amorphization of PANI may occur during CG process; the other is that the surface of rGO has been covered with a large amount of small PANI granules.
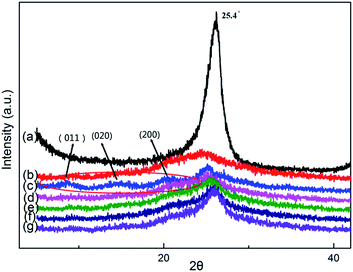 | ||
| Fig. 3 X-ray diffraction of (a) rGO, (b) CGed–PANI, (c) PANI, (d) CGed–PANI/(5 wt%)rGO, (e) CGed–PANI/(10 wt%)rGO, (f) CGed–PANI/(20 wt%)rGO, (g) CGed–PANI/(30 wt%)rGO. | ||
The XPS spectra of GO, rGO and CGed–rGO(5 wt%)/PANI are shown in Fig. 4. The core-level XPS signal of C 1s for GO and rGO exhibit a main peak centered at about 284.6 eV originating from the graphitic sp2 carbon atoms. The weak peaks located at 286.4 eV are due to carbon atoms connecting with oxygenate groups, such as C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.31,39 Moreover, as is shown, the peak at 284.6 eV of rGO is much longer than the peak of GO, and the peak at 286.4 eV of GO is much broader than the peak of rGO.32 GO has a strong oxygen peak representing an oxygen atomic content about 30.28%, higher than that in the rGO (7.27%). As shown in Fig. 4d, the C 1s core-level spectrum of as-synthesized rGO–PANI composites can be curve-fitted into five peak components: carbon sp2 at 284.6 eV, carbon sp3 at 285.3 eV, C–N group at 285.9 eV, C–O group at 287.0 eV and C
O.31,39 Moreover, as is shown, the peak at 284.6 eV of rGO is much longer than the peak of GO, and the peak at 286.4 eV of GO is much broader than the peak of rGO.32 GO has a strong oxygen peak representing an oxygen atomic content about 30.28%, higher than that in the rGO (7.27%). As shown in Fig. 4d, the C 1s core-level spectrum of as-synthesized rGO–PANI composites can be curve-fitted into five peak components: carbon sp2 at 284.6 eV, carbon sp3 at 285.3 eV, C–N group at 285.9 eV, C–O group at 287.0 eV and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group at 288.5 eV, implying that the C
O group at 288.5 eV, implying that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of reduced graphene oxide were doped into PANI.45 The interaction between the PANI backbone and reduced graphene oxide sheets results in the increased conjugation and shift in binding energy. The N 1s core-level spectrum of as-synthesized rGO/PANI composites can be curve-fitted into three peak components with BEs at 398.2 eV, 399.3 eV and >400 eV, attributable to the amine (–NH–), imine (
O groups of reduced graphene oxide were doped into PANI.45 The interaction between the PANI backbone and reduced graphene oxide sheets results in the increased conjugation and shift in binding energy. The N 1s core-level spectrum of as-synthesized rGO/PANI composites can be curve-fitted into three peak components with BEs at 398.2 eV, 399.3 eV and >400 eV, attributable to the amine (–NH–), imine (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), and positively charged nitrogen (N+, >400 eV) species, respectively.46 As compared with the XPS of PANI, the nitrogen content is decreased in the rGO/PANI composites. All the above information indicates that rGO doping takes place on the quinoid segment of PANI after the cryogenic grinding process.
N–), and positively charged nitrogen (N+, >400 eV) species, respectively.46 As compared with the XPS of PANI, the nitrogen content is decreased in the rGO/PANI composites. All the above information indicates that rGO doping takes place on the quinoid segment of PANI after the cryogenic grinding process.
 | ||
| Fig. 4 C 1s XPS spectra of (a) GO, (b) rGO, (c) PANI, and (d) N 1s XPS spectra of CGed–(5 wt%)rGO/PANI. | ||
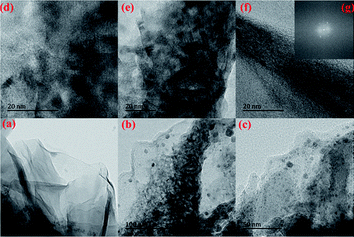
The typical morphology of the resulting rGO and the hybrid CGed–PANI/(5 wt%)rGO composites are observed by TEM, as shown in Fig. 5. The typical wrinkled layer morphology of the rGO is given in Fig. 5a, which shows the layer-by-layer structure and network structure of rGO. For CGed–PANI/(5 wt% rGO) composite in Fig. 5b–g, we can see the CGed–PANI are homogeneously dispersed on the rGO sheets. PANI shows granular and rod-like morphology with 20–60 nm. The selected area electron diffraction pattern in inset of Fig. 5f shows that PANI lacks obvious crystalline character and the rGO prepared here has a typically curved, good crystalline character. Because a serious brittle fracturing of the PANI powder could occur in the liquid nitrogen at −195.6 °C. Furthermore, grinding is favorable for polymer chain fission, cross-linking and increase in the amorphous content. We can see the ordered molecular structure PANI on the surface of reduced grapheme oxide layer after CG progress. TEM images show that the uniform PANI mainly disperses on the surface or intercalates between the rGO sheets because of the strong π–π interactions. The homogeneous dispersion of PANI on rGO sheet indicates that the cryogenic grinding is a simple, effective and low-cost method.
Thermoelectric properties
The thermoelectric properties of the composites were measured in the temperature range from 300 K to 380 K, as shown in Fig. 6a–e. It can be seen that the electrical conductivity of the composites increases dramatically as the rGO content increases, and reaches 1868.8 S m−1 for the sample with rGO content of 5% at 300 K, which is larger than the pure PANI. The enhancement of the electrical conductivity should be attributed to the strong π–π interaction between π-bonded surface of the rGO and the conjugated structure of polyaniline. This behavior can be explained by the template of reduced graphene oxide created by the rGO and excellent electrical performance of rGO (graphenes have high carrier mobility in excess of 105 cm2 V−1 s−1, high thermal conductivity up to 5000 W m−1 K−1 and high electrical conductivity about 106 S m−1).19,20 Furthermore, the Seebeck coefficient shows great increase from 5.3 to 15.9 μV K−1 when the rGO content changes from 0 to 10 wt%. The positive Seebeck coefficient indicates that the composite is a p-type semiconductor. The rGO content dependence of the power factor is shown in Fig. 6c. It can be seen that the power factor increases obviously from 1.1 × 10−8 W m−1 K−2 to 42.8 × 10−8 W m−1 K−2 with the increase of rGO from 0 to 10%. But, both the electrical conductivity and Seebeck coefficient are slightly reduced with rGO content ranging from 5% to 30%. The possible reason may be that the rGO is not reduced absolutely (as shown in the elemental composite (c) of Table 1), and oxygen-containing groups of rGO may hinder the carrier transport. Furthermore, all samples exhibit low thermal conductivity values in the range of 0.296–0.566 W m−1 K−1 in Fig. 6d. But, the thermal conductivity values of the rGO/composites slightly increase with rGO content ranging from 5% to 30%. rGO sheets have higher thermal conductivity values about (4.84 ± 0.44)–(5.30 ± 0.48) × 10−3 W m−1 K−1 than PANI, so the thermal conductivity values of composites increases with rGO content.51–55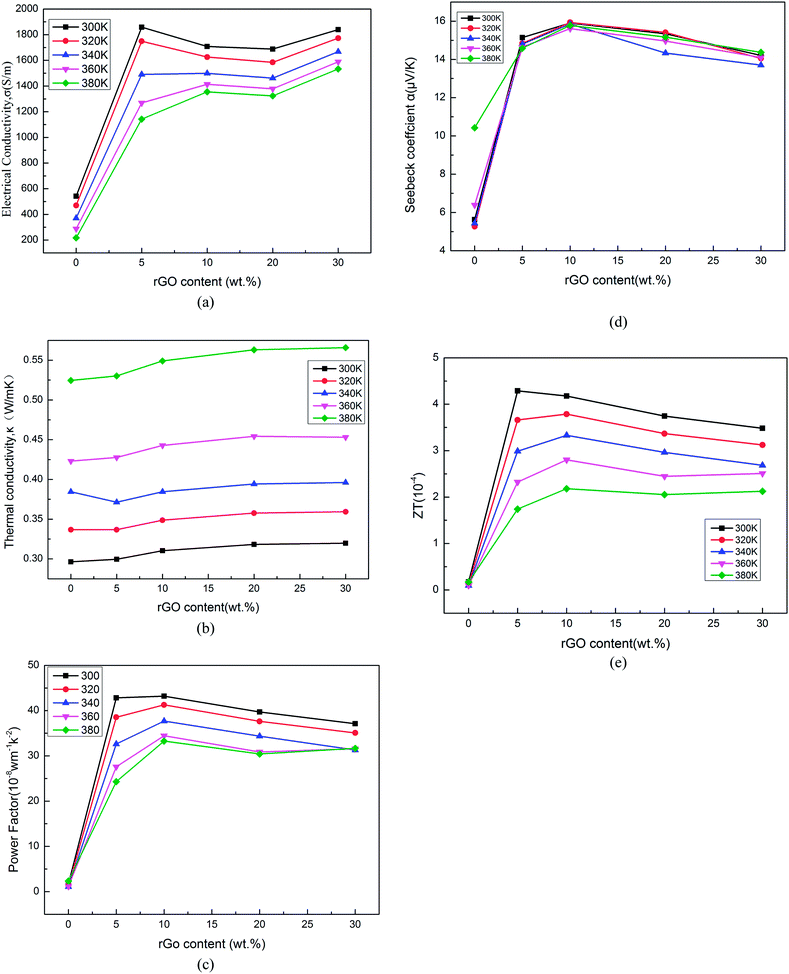 | ||
| Fig. 6 Electrical conductivity (a), Seebeck coefficient (b), power factor (c), thermal conductivity values (d) and the ZT value (e) of rGO/PANI hybrid composites with different contents of rGO. | ||
| C | O | N | |
|---|---|---|---|
| (a) PANI | 80.25% | 10.04% | 6.44% |
| (b) GO | 64.56% | 30.28% | — |
| (c) rGO | 89.22% | 7.27% | — |
| (d) CGed–(5 wt%)rGO/PANI | 88.26% | 7.00% | 3.32% |
To clarify the reason for the changes in electrical conductivity, we measured the Hall coefficient (RH) at room temperature on a PPMS system and calculated the carried concentrations, assuming parabolic bands and a single band conduction process. As shown in Table 2, when the rGO content changes from 0 to 5 wt%, the carrier mobility increases greatly. It is also found that the carrier concentration shows little change with the increase of rGO content. This also plays a major role in the increase of Seebeck coefficient. As is known, interchain and intrachain hopping have important effects on the charge carrier transport in polymers. The carrier mobility is strongly dependent on the conformation and arrangement of polymer chains. After CG process, the size of PANI gets smaller. The rGO becomes a template for conductive PANI and a wonderfully conductive medium. These highly oriented polymer chains can reduce the barriers of interchain and intrachain hopping and allow the carrier to move easily. Typically, the electrical conductivity of inorganic semiconductor TE materials rises with the increasing carrier concentration, but on the contrary, the Seebeck coefficient tends to decrease at the same time.57 However, the Seebeck coefficient of polymer thermoelectric materials often rises even when the electrical conductivity increases. We can see the same changing trend in some other PANI systems, such as PANI/Te composite, PANI/SWNT, PANI/GO, and others.58–61 In our work, polyaniline molecules ordered on the rGO surface with the induction of rGO and π–π conjugate effect between the PANI and rGO. Thus, the carrier concentration of composite increased with 5% rGO. However, with more rGO added, the oxygen-containing groups on the surface of the rGO increase the barriers of interchain and intrachain. In addition, some electrons become “cold” electrons with energy below the Fermi level of the PANI/rGO composite. Thus, the carrier concentration decreased with more added rGO.58 Concomitantly, the carrier mobility increases, resulting in increased electrical conductivity.15,56
| Sample | Carrier concentration (cm−3) | Carrier mobility (cm2 V−1 s−1) |
|---|---|---|
| PANI | 4.9 × 1021 | 0.69 |
| 5% rGO/PANI | 9.8 × 1021 | 1.38 |
| 10% rGO/PANI | 8.5 × 1021 | 1.09 |
| 20% rGO/PANI | 8.9 × 1021 | 1.18 |
| 30% rGO/PANI | 7.9 × 1021 | 1.21 |
The highest ZT value is 4.29 × 10−5 at 300 K for the 5 wt% sample. The enhancement of the thermoelectric performance should be attributed to the interaction between PANI and the rGO. Compared with the in situ polymerization method, cryogenic grinding has obvious advantages in improving the degree of dispersion of the rGO. The increased contact area strengthens the conjugation between the rGO and PANI molecules, and lowers the carrier hopping barrier. After CG progress, the PANI particles dispersed on the rGO sheet, increasing the carrier transport as a conducting bridge.
Conclusions
The rGO/PANI composites have been prepared through a cryogenic grinding technique combined with SPS. The results show that the refined PANI particles are homogeneously dispersed and orderly arranged on the rGO templates, which gives rise to the simultaneous enhancement of carrier concentration and carrier mobility. As the rGO content increases from 0% to 30%, the electrical conductivity of rGO/PANI increase from 217 S m−1 to 1869 S m−1, and the thermal conductivity shows little change. On the other hand, rGO are been uniformly dispersed in the composites after CG process. The rGO has two important roles both as conducting medium and the template. Consequently, the maximum ZT of 4.29 × 10−4 is found at 300 K for the PANI sample with 5 wt% rGO, much higher than that of the PANI without rGO (ZT = 1.73 × 10−5), which suggests the superiority of CG technique for the dispersion of PANI and the high efficiency of rGO on enhancing thermoelectric properties. These results demonstrate that the addition of rGO by cryogenic grinding is an effective way for improving the thermoelectric properties of PANI, which could be applicable to many other thermoelectric materials. Finally, as for bulk materials, the ZT value of 4.29 × 10−4 is not very good compared with film materials. Nevertheless, bulk materials are more conducive for preparation of thermoelectric devices with various shapes.Therefore, it is expected to further improve the thermoelectric performance through further optimization of doping level and the microstructure of polyanilin.56 Moreover, the ZTs of our composites are still not too high, therefore it is necessary to improve the degree of order of polyaniline polymer to enhance the ZT.
Acknowledgements
This work was funded by Natural Science Foundation of China (no. 51374078 and 51403037), Shanghai Committee of Science and Technology (no. 13JC1400100 and 13NM1400101), PCSIRT (no. IRT1221), the Fundamental Research Funds for the Central Universities and DHU Distinguished Young Professor Program.References
- H.-K. Lyeo, A. Khajetoorians, L. Shi, K. P. Pipe, R. J. Ram and A. Shakouri, et al., Science, 2004, 303(5659), 816–818 CrossRef CAS PubMed.
- A. Minnich, M. Dresselhaus, Z. Ren and G. Chen, Energy Environ. Sci., 2009, 2(5), 466–479 CAS.
- G. S. Nolas, J. Sharp and H. J. Goldsmid, Thermoelectrics: basic principles and new materials developments, Springer, 2001 Search PubMed.
- J. P. Heremans, V. Jovovic, E. S. Toberer, A. Saramat, K. Kurosaki and A. Charoenphakdee, et al., Science, 2008, 321(5888), 554–557 CrossRef CAS PubMed.
- Y. Min, J. W. Roh, H. Yang, M. Park, S. I. Kim and S. Hwang, et al., Adv. Mater., 2013, 25(10), 1425–1429 CrossRef CAS PubMed.
- Q. Zhang, X. Ai, W. Wang, L. Wang and W. Jiang, Acta Mater., 2014, 73, 37–47 CrossRef CAS PubMed.
- M. S. Toprak, C. Stiewe, D. Platzek, S. Williams, L. Bertini and E. C. Muller, et al., Adv. Funct. Mater., 2004, 14(12), 1189–1196 CrossRef CAS.
- L.-D. Zhao, S.-H. Lo, Y. Zhang, H. Sun, G. Tan and C. Uher, et al., Nature, 2014, 508(7496), 373–377 CrossRef CAS PubMed.
- O. Bubnova, Z. U. Khan, A. Malti, S. Braun, M. Fahlman and M. Berggren, et al., Nat. Mater., 2011, 10(6), 429–433 CrossRef CAS PubMed.
- C. Yoon, M. Reghu, D. Moses, A. Heeger, Y. Cao and T.-A. Chen, et al., Synth. Met., 1995, 75(3), 229–239 CrossRef CAS.
- F. Yakuphanoglu and B. Senkal, J. Phys. Chem. C, 2007, 111(4), 1840–1846 CAS.
- Y. Du, S. Z. Shen, K. Cai and P. S. Casey, Prog. Polym. Sci., 2012, 37(6), 820–841 CrossRef CAS PubMed.
- F. Yakuphanoglu and B. F. Senkal, J. Phys. Chem. C, 2007, 111(4), 1840–1846 CAS.
- C. G. Wu, D. C. DeGroot, H. O. Marcy, J. L. Schindler, C. R. Kannewurf and Y. J. Liu, et al., Chem. Mater., 1996, 8(8), 1992–2004 CrossRef CAS.
- Y. Zhao, G.-S. Tang, Z.-Z. Yu and J.-S. Qi, Carbon, 2012, 50(8), 3064–3073 CrossRef CAS PubMed.
- Q. Zhang, W. Wang, J. Li, J. Zhu, L. Wang and M. Zhu, et al., J. Mater. Chem. A, 2013, 1(39), 12109–12114 CAS.
- W. Qun, Y. Qin, C. Jiang and C. Lidong, J. Mater. Chem., 2012, 22(34), 17612–17618 RSC.
- J. Xiang and L. T. Drzal, Polymer, 2012, 53(19), 4202–4210 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang and S. Dubonos, et al., Science, 2004, 306(5696), 666–669 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mat., 2007, 6(3), 183–191 CrossRef CAS PubMed.
- J. S. Bunch, A. M. van der Zande, S. S. Verbridge, I. W. Frank, D. M. Tanenbaum and J. M. Parpia, et al., Science, 2007, 315(5811), 490–493 CrossRef CAS PubMed.
- I. V. Lightcap, T. H. Kosel and P. V. Kamat, Nano Lett., 2010, 10(2), 577–583 CrossRef CAS PubMed.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3(5), 270–274 CrossRef CAS PubMed.
- L. Tapaszto, G. Dobrik, P. Lambin and L. P. Biro, Nat. Nanotechnol., 2008, 3(7), 397–401 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai and P. J. Ferreira, et al., Science, 2011, 332(6037), 1537–1541 CrossRef CAS PubMed.
- E. Yoo, J. Kim, E. Hosono, H.-s. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8(8), 2277–2282 CrossRef CAS PubMed.
- X. Wang, L. Zhi and K. Muellen, Nano Lett., 2008, 8(1), 323–327 CrossRef CAS PubMed.
- M. Kim, C. Lee and J. Jang, Adv. Funct. Mater., 2014, 24(17), 2489–2499 CrossRef CAS.
- A. C. Neto, F. Guinea, N. Peres, K. S. Novoselov and A. K. Geim, Rev. Mod. Phys., 2009, 81(1), 109 CrossRef.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4(4), 1963–1970 CrossRef CAS PubMed.
- K. Zhang, L. L. Zhang, X. S. Zhao and J. Wu, Chem. Mater., 2010, 22(4), 1392–1401 CrossRef CAS.
- N. A. Kumar, H.-J. Choi, Y. R. Shin, D. W. Chang, L. Dai and J.-B. Baek, ACS Nano, 2012, 6(2), 1715–1723 CrossRef CAS PubMed.
- L. Al-Mashat, K. Shin, K. Kalantar-Zadeh, J. D. Plessis, S. H. Han and R. W. Kojima, et al., J. Phys. Chem. C, 2010, 114(39), 16168–16173 CAS.
- J. Huang, J. A. Moore, J. H. Acquaye and R. B. Kaner, Macromolecules, 2005, 38(2), 317–321 CrossRef CAS.
- Y. Long, Z. Chen, X. Zhang, J. Zhang and Z. Liu, Appl. Phys. Lett., 2004, 85(10), 1796–1798 CrossRef CAS PubMed.
- A. G. MacDiarmid and A. J. Epstein, Synth. Met., 1995, 69(1), 85–92 CrossRef CAS.
- S. Park, J. An, R. D. Piner, I. Jung, D. Yang and A. Velamakanni, et al., Chem. Mater., 2008, 20(21), 6592–6594 CrossRef CAS.
- W. Gao, L. B. Alemany, L. Ci and P. M. Ajayan, Nat. Chem., 2009, 1(5), 403–408 CrossRef CAS PubMed.
- C. Botas, P. Álvarez, P. Blanco, M. Granda, C. Blanco and R. Santamaría, et al., Carbon, 2013, 65, 156–164 CrossRef CAS PubMed.
- P. Yu, Y. Li, X. Zhao, L. Wu and Q. Zhang, Langmuir, 2014, 30(18), 5306–5313 CrossRef CAS PubMed.
- T. Lindfors and R.-M. Latonen, Carbon, 2014, 69, 122–131 CrossRef CAS PubMed.
- J. Yan, T. Wei, B. Shao, Z. Fan, W. Qian and M. Zhang, et al., Carbon, 2010, 48(2), 487–493 CrossRef CAS PubMed.
- C. Valles, P. Jimenez, E. Munoz, A. M. Benito and W. K. Maser, J. Phys. Chem. C, 2011, 115(21), 10468–10474 CAS.
- X. Huang, N. Hu, R. Gao, Y. Yu, Y. Wang and Z. Yang, et al., J. Mater. Chem., 2012, 22(42), 22488–22495 RSC.
- L. Mi, H. Xingyi, W. Chao, X. Haiping, J. Pingkai and T. Tanaka, J. Mater. Chem., 2012, 22(44), 23477–23484 RSC.
- J. Lu, W. Liu, H. Ling, J. Kong, G. Ding and D. Zhou, et al., RSC Adv., 2012, 2(28), 10537–10543 RSC.
- P. Liu, Y. Huang, L. Wang and W. Zhang, Synth. Met., 2013, 177, 89–93 CrossRef CAS PubMed.
- N. A. Kumar, H.-J. Choi, Y. R. Shin, D. W. Chang, L. Dai and J.-B. Baek, ACS Nano, 2012, 6(2), 1715–1723 CrossRef CAS PubMed.
- W. Wang, S. Sun, S. Gu, H. Shen, Q. Zhang and J. Zhu, et al., RSC Adv., 2014, 4(51), 26810–26816 RSC.
- H. Wang, Q. Hao, X. Yang, L. Lu and X. Wang, Nanoscale, 2010, 2(10), 2164–2170 RSC.
- J. H. Seol, I. Jo, A. L. Moore, L. Lindsay, Z. H. Aitken and M. T. Pettes, et al., Science, 2010, 328(5975), 213–216 CrossRef CAS PubMed.
- H. Yan, T. Ohta and N. Toshima, Macromol. Mater. Eng., 2001, 286, 139–142 CrossRef CAS.
- H. Yan, N. Sada and N. Toshima, J. Therm. Anal. Calorim., 2002, 69, 881–887 CrossRef CAS.
- R. Islam, R. Chan-Yu-King, J.-F. Brun, C. Gors, A. Addad, M. Depriester, A. Hadj-Sahraoui and F. Roussel, Nanotechnology, 2014, 25, 475705 CrossRef PubMed.
- J. Choi, N. D. Tu, S.-S. Lee, H. Lee, J. S. Kim and H. Kim, Macromol. Res., 2014, 22, 1104–1108 CrossRef CAS PubMed.
- Q. Yao, L. Chen, W. Zhang, S. Liufu and X. Chen, ACS Nano, 2010, 4, 2445–2451 CrossRef CAS PubMed.
- D. M. Rowe, CRC handbook of thermoelectrics, CRC press, 1995 Search PubMed.
- N. E. Coates, S. K. Yee, B. McCulloch, K. C. See, A. Majumdar, R. A. Segalman and J. J. Urban, Adv. Mater., 2013, 25, 1629–1633 CrossRef CAS PubMed.
- Q. Yao, Q. Wang, L. Wang, Y. Wang, J. Sun, H. Zeng, Z. Jin, X. Huang and L. Chen, J. Mater. Chem. A, 2014, 2, 2634–2640 CAS.
- Q. Yao, Q. Wang, L. Wang and L. Chen, Energy Environ. Sci., 2014, 7, 3801–3807 CAS.
- Y. Zhao, G.-S. Tang, Z.-Z. Yu and J.-S. Qi, Carbon, 2012, 50, 3064–3073 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |