Anion-directed assembly of helical copper(II) complexes based on a bispyridylpyrrole ligand: synthesis, structural and magnetic properties†
Wen-Zhong Fangab,
Ya-Ping Wangab,
Yi-Fan Wanga,
Shou-Chun Zhanga and
Xiao-Yi Yi*abc
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan 410083, P. R. China. E-mail: xyyi@mail.csu.edu.cn; Fax: +86 731 88879616; Tel: +86 731 88879616
bInnovation Base of Energy and Chemical Materials for Graduate Students Training, Central South University, Changsha, Hunan 410083, P. R. China
cState Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin 300071, P. R. China
First published on 23rd December 2014
Abstract
Helical copper(II) complexes based on a bispyridylpyrrole ligand, namely the helical polymer {[Cu2(PDPH)2(N3)2]}n (1) (where HPDPH = 2,5-bis(2′-pyridyl)pyrrolide) containing a one-dimensional double-helical chain, a discrete double-helical complex [Cu2(PDPH)2(NO3)2] (2) and triple-helical complex [Cu2(PDPH)3](OTf) (3·OTf) (where OTf− = triflato) were synthesized by displacement of Cl− in [Cu(PDPH)Cl] by the anion N3−, NO3− and OTf−, respectively. The structures of these complexes directly correlate with the coordination abilities of the ligands. The N3− anion is found to favor the formation of polymeric helical structures. The helical chain in 1 is built-up by [Cu2(PDPH)2] units linked by double μ1,3-azido bridges. In 2, the anion NO3− acting as a monodentate oxygen ligand binds to each copper center, resulting in the formation of the double helical structure, while the non-coordinating OTf− ligand leaves space for three PDPH− ligands, leading to the formation of the triple-helical dicopper complex 3·OTf. Magnetic susceptibility data of 1, measured from 1.8 to 300 K, show alternating ferro- and antiferromagnetic interactions through the bridging PDPH− and μ1,3-azido pathway, respectively.
Introduction
Transition metal helicates have a wide range of interesting applications including catalysis, probing DNA structures, optical and magnetic materials.1–7 Numerous examples of metal helicates have been described in the literature so far. They can be single-, double- and triple-helical. The flexible multidentate ligand plays a crucial role in the specific formation of a versatile helical structure. Preorganized linear oligonitrogen donors, linear oligooxygen donors, mixed oxygen/nitrogen donors and ligands possessing sulfur, carbon or phosphorous donors are effective in producing helicates.8–12 Anion-directed assembly is common strategy for the synthesis of coordination polymer complexes.13–16 Anion can directly influence the structure by coordinating to the metal ions or by acting as templates to induce the self-assembly. However, to our knowledge, the examples of metal helicates depending on the anion present during self-assembly process are relative rare.17–20 For example, a family of circular double helicates from the reaction of tris-bipy ligand and iron(II) salts were obtained under the exact same conditions, the only difference residing in the anion present during the helicate formation.17,18We have a long-standing interest in the metal complexes based on mono-anionic tridentate 2,5-bis(2′-pyridyl)pyrrolide (PDPH−) ligand (Scheme 1). Not only this N3 ligand has the π-backbonding capability, but also pyrrolate donor with flexible π-properties can behave as bridging ligand due to its versatile π-donor and π-acceptor responding to the metal site π-bonding properties.21–24 PDPH− ligand is usually recognized as an analogue of the neutral terpyridine ligand (tpy). A number of helical Cu(I) complexes with tpy or its derivatives are reported. It inspired us to pursue new helical copper complexes with anionic bispyridylpyrrole ligand. In our previous studies, a hetero-sodium/copper(I) complex with PDPH− ligand in a helical arrangement around the copper(I) has been characterized.24 Obviously, PDPH− is a suitable candidate for synthesis of helical metal complexes.
Thus, we will describe the preparation, crystal structures of a series of copper(II) helicates based on bispyridylpyrrole ligand. They are {[Cu2(PDPH)2(N3)2]}n (1) with one-dimensional double-helical chain, discrete double-helical [Cu2(PDPH)2(NO3)2] (2) and triple-helical [Cu2(PDPH)3](OTf) (3·OTf) obtained from the reactions of [Cu(PDPH)Cl]2 with NaN3, NaNO3 and AgOTf (where OTf− = triflato), respectively. Primary magnetic result of 1 is also presented.
Experimental sectoins
General considerations
All manipulations were carried out under nitrogen by standard Schlenk techniques unless otherwise stated. Solvents were purified, distilled and degassed prior to use. Infrared spectra (KBr) were recorded on a Perkin-Elmer 16 PC FT-IR spectrophotometer. Elemental analyses were performed by a PE240C elemental analyzer. The magnetic susceptibility data were performed on a Quantum Design MPMS-XL7 SQUID magnetometer. The starting 2,5-bis(2′-pyridyl)pyrrolide (HPDPH)25 and [Cu(PDPH)Cl]26 were prepared according to literature methods. All of other chemicals were obtained from J&K Scientific Ltd.Synthesis of {[Cu2(PDPH)2(N3)2]}n (1)
A mixture of [Cu(PDPH)Cl] (64 mg, 0.2 mmol) and NaN3 (13 mg, 0.2 mmol) in THF was stirred overnight and filtered. The filtrate was layered with hexane to give green needle crystals which were suitable for X-ray diffraction study. Yield: 54 mg (83%). IR (KBr, cm−1): 3069(w), 3022(w), 2052(vs), 1600(s), 1552(m), 1516(m), 1449(m), 1436(s), 1377(w), 1337(s), 1260(m), 1144(m), 1121(w), 1051(m), 1004(m), 955(w), 743(s), 708(w), 690(w), 645(w). Anal. calcd for C28H20Cu2N12·1.2(thf): C, 53.50; H, 4.11; N, 22.55. Found: C, 53.98; H, 4.03; N, 22.89.Synthesis of [Cu2(PDPH)2(NO3)2] (2)
A mixture of [Cu(PDPH)Cl] (64 mg, 0.2 mmol) and NaNO3 (17 mg, 0.2 mmol) in THF was stirred overnight and filtered. The filtrate was layered with hexane to give green block crystals which were suitable for X-ray diffraction study. Yield: 60 mg (87%). IR (KBr, cm−1): 3083(w), 3056(w), 1602(s), 1558(w), 1532(m), 1508(m), 1486(s), 1449(s), 1436(s), 1376(w), 1340(m), 1287(s), 1264(s), 1152(m), 1122(w), 1082(w), 1043(m), 1010(s), 954(w), 756(s), 710(w), 686(w), 647(w). Anal. calcd for C28H20Cu2N8O6·0.3(thf): C, 49.17; H, 3.17; N, 15.71. Found: C, 49.12; H, 3.05; N, 15.89.Synthesis of [Cu2(PDPH)3]·OTf (3·OTf)
A mixture of [Cu(PDPH)Cl] (128 mg, 0.4 mmol) and AgOTf (104 mg, 0.4 mmol) in THF was stirred overnight and filtered. The filtrate was layered with hexane to give green block crystals which were suitable for X-ray diffraction study. Yield: 78 mg (62%). IR (KBr, cm−1): 3089(w), 3031(w), 1602(s), 1558(m), 1506(s), 1453(m), 1434(s), 1389(w), 1332(s), 1259(s), 1223(m), 1147(s), 1100(w), 1052(m), 1030(s), 953(w), 785(m), 753(s), 712(m), 638(m). Anal. calcd for C43H30Cu2F3N9O3S: C, 55.12; H, 3.23; N, 13.45. Found: C, 55.32; H, 3.45; N, 13.51.X-ray crystallography
Diffraction data of 1–3·OTf were recorded on a Bruker CCD diffractometer with monochromatized Mo-Kα radiation (λ = 0.71073 Å). The collected frames were processed with the software SAINT. The absorption correction was treated with SADABS.27 Structures were solved by direct methods and refined by full-matrix least-squares on F2 using the SHELXTL software package.28 Atomic positions of non-hydrogen atoms were refined with anisotropic parameters. All hydrogen atoms were introduced at their geometric positions and refined as riding atoms. In complex 1, one tetrahydrofuran molecule is co-crystallized.Results and discussion
Synthesis and characterization
Replacements of Cl− ligand in [Cu(PDPH)Cl] by N3−, NO3− and OTf− give helical polymer {[Cu2(PDPH)2(N3)2]}n (1), discrete double-helical [Cu2(PDPH)2(NO3)2] (2) and triple-helical [Cu2(PDPH)3](OTf) (3·OTf) in good yields and excellent purity, respectively (Scheme 2). 1–3·OTf are readily soluble in common organic solvents, such as CH2Cl2, THF and DMSO, yet not soluble in water and nonpolar solvents such as diethyl ether and hexane. These complexes are air-stable in solid state and in solution. Recrystallization of the crude products from tetrahydrofuran by diffusion of hexane afford crystals which are suitable for X-ray diffraction study. The IR spectra of 1–3·OTf indicate that the PDPH− ligand has several distinctive signals, including weak aromatic C–H stretching bands at about 3022–3089 cm−1, and moderately strong stretching bands at 1340–1600 cm−1 corresponding to the in-plane vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, which are well comparable with those in the coinage metal complexes with PDPH− ligand.24 A strong stretching band at 2052 cm−1 in 1 due to azido groups, as well as strong signals at 1010 and 1030 cm−1 attributed to nitrato and triflato ligands in complexes 2 and 3·OTf, respectively, are observed.
N bonds, which are well comparable with those in the coinage metal complexes with PDPH− ligand.24 A strong stretching band at 2052 cm−1 in 1 due to azido groups, as well as strong signals at 1010 and 1030 cm−1 attributed to nitrato and triflato ligands in complexes 2 and 3·OTf, respectively, are observed.
Structure description
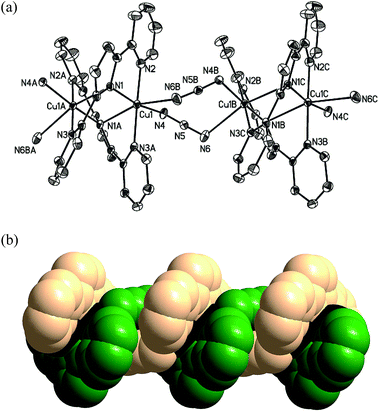
The structures of 1–3·OTf are confirmed by single crystal X-ray diffraction method. A summary of crystallographic data and experimental details for these complexes are given in Table 1. The ORTEP diagrams for 1–3+ with ellipsoids and their space-filling representation are shown in Fig. 1–3, respectively.| 1·thf | 2 | 3·OTf | |
|---|---|---|---|
| a GoF = [∑w(|Fo| − |Fc|)2/(Nobs − Nparam)]1/2.b R1 = ∑||Fo| − |Fc||/∑|Fo|.c wR2[(∑w|Fo| − |Fc|)2/∑w2|Fo|2]1/2. | |||
| Formula | C32H28Cu2N12O | C14H10CuN4O3 | C43H30Cu2F3N9O3S |
| Fw | 723.74 | 345.80 | 936.90 |
| Crystal system | Orthorhombic | Monoclinic | Orthorhombic |
| Space group | Pbcn | C2/c | Pca2(1) |
| a, Å | 8.22470(10) | 17.6233(3) | 18.6566(9) |
| b, Å | 33.0397(5) | 12.3436(2) | 11.8775(6) |
| c, Å | 11.3562(2) | 15.2939(3) | 19.2374(10) |
| β, degree | 123.0260(10) | ||
| V, Å3 | 3085.95(8) | 2789.40(9) | 4262.9(4) |
| Z | 4 | 8 | 4 |
| ρcalc, g cm−3 | 1.558 | 1.647 | 1.460 |
| T, K | 293(2) | 293(2) | 293(2) |
| μ, mm−1 | 1.428 | 1.584 | 1.110 |
| No. of refln | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 889 889 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205 205 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 751 |
| No. of indep. refln | 3583 | 3199 | 9179 |
| Rint | 0.0246 | 0.0146 | 0.0581 |
| GoFa | 1.081 | 1.085 | 1.013 |
| R1b, wR2c [I > 2σ(I)] | 0.0358, 0.0827 | 0.0205, 0.0645 | 0.0647, 0.1546 |
| R1, wR2 (all data) | 0.0522, 0.0976 | 0.0215, 0.0654 | 0.1053, 0.1685 |
Known from our previous studies,26 starting material [Cu(PDPH)Cl] is mononuclear. Its structure is analogous with that of known bispyridylpyrrolide metal complexes, such as [M(PDPH)Cl] (M = Pd, Pt).22 The total bond angels around Cu is 359.42°, which is close approximately to 360°, indicating of a planar structure. Three interplanar angles among two side rings and the pyrrole ring are close to zero. The bond distance of Cu–Cl is 2.2216(11) Å. Its Cu–Npyridine distance (2.141(3) Å and 2.148(3) Å) is longer than that of Cu–Npyrrole (1.862(3) Å).
As shown in Fig. 1a, the structural feature of complex 1 is one dimensional double-helical chain. Two PDPH− ligands are twisted around Cu⋯Cu axis to form [Cu2(PDPH)2]2+ building block. Two μ-1,3 N3− ligands bridge the [Cu2(PDPH)2]2+ building block to generate neutral 1D helical coordination polymer. The analogous polymeric helical chains containing copper(I) terpyridine units [Cu2(terpy)2]2+ (where terpy = 2,2′:6′2′′-terpyridine)29 was reported, where [Cu2(terpy)2]2+ building block is linked through weak Cu(I)–Cu(I) d10–d10 secondary bonds.
In the [Cu2(PDPH)2]2+ building block of 1, each Cu atom locates in the center of elongated octahedron geometry. Two long axial sites are provided by N1A from equivalent PDPH− and N6B from the azido ligand with distances of 2.534(3) and 2.624(3) Å, respectively. The bond angle of N1A–Cu1–N6B is 161.1(8)°. The basal plane is defined by the N2, N1, N3A from two PDPH− ligands and N4 from azido ligand. The Cu1–N1, Cu1–N2, Cu1–N3A and Cu1–N4 bond distances are 2.002(2), 2.037(2), 2.0555(19) and 1.977(2) Å. To be notable, the central N atom of each N3− ligand lies close to a Cu(II) with a distance of 2.768(2) Å, comparable with that of a weak Cu(II)–N bond. The Cu1–Cu1A seperation (3.062(6) Å) is longer than Cu1–Cu1B (5.163(6) Å). The angle of Cu1–N1–Cu1A is 84.1(3)°.
Complex 2 is a discrete double-helicate. Each copper(II) in 2 is in an irregular five-coordinate environment to form distorted trigonal dipyramidal geometry with four short contacts to one oxygen atom from NO3− (Cu1–O2 2.0104(10) Å), two nitrogen atom from a “monopyridylpyrrole” fragment of one PDPH− ligand (Cu1–N2A 1.9878(12) Å; Cu1–N3A 2.0035(11) Å) and one nitrogen atom from a terminal pyridine of the other PDPH− ligand (Cu1–N1 2.0194(10) Å). The coordination sphere is completed by a long contact to the pyrrole of the second PDPH− ligand (Cu1–N2 2.3712(12) Å). The copper–copper distance is 2.9234(3) Å. The metal ions in the helicates commonly are four-, six- or eight-coordinate, but cases of helicate with metal maintaining a trigonal dipyramidal geometry as 2 are very rare. The only known example is a helical oligopyridine palladium complex.11
The crystal structure determination shows 3·OTf is composed of [Cu2(PDPH)3]+ (3+) cation and an uncoordinated triflate anion. 3+ displays an unusual triple-helical structure in which three PDPH− ligands wrap in a helical arrangement around two copper atoms. Each Cu atom is also penta-coordinate by five nitrogen atoms from three dipyridinepyrrole ligands to form trigonal dipyramidal geometry. N3, N5, N9 around Cu1 as well as N1, N5, N7 around Cu2 atoms form the basal plane, and N4, N8 and N2, N6 atoms occupy their respective axial direction. The average distance of Cu–N on the basal plane (av. 2.151 Å) is longer than that of Cu–N on the axis (av. 1.969 Å). The N4–Cu1–N8 and N2–Cu2–N6 on the axis are nearly linear with bond angles of 176.1(2)° and 170.8(2)°, respectively. The Cu⋯Cu seperation of 2.8147(10) Å in 3+ is significantly shorter than that of 1 and 2, perhaps due to one more PDPH− ligand chelating two copper atoms.
Structural investigations of complexes 1-3+ reveal that the bispyridylpyrrole ligand is flexible and is capable of acting as chelating and bridging ligand to bound to Cu(II) atom in bonding mode of μ2-(κ2-N,N′), (κ2-N′,N′′) and/or μ2-(κ2-N,N′), N′′. The PDPH− ligands in these complexes are twisted. Three interplanar angles with the central ring are in the range of 4.0(1)–35.8(1)°, well comparable with those found in the coinage metal terpyridine and pyridylpyrrole complexes.24,30,31
Magnetic properties of complex 1
Variable-temperature magnetic susceptibility measurement was performed on crystalline 1 in the range of 1.8–300 K at 1000 G. The χMT versus T plots and 1/χM versus T plots are shown in Fig. 4. The effective magnetic moment (2.828μB per Cu2) at 300 K is well consistant with the theoretical value (2.828μB) expected for a two uncoupled S = 1/2 spin system. The χMT value continuously increases and reaches 1.41 cm3 K mol−1 at 3.0 K on cooling from room temperature, indicating of ferromagnetic interaction between the Cu(II) ions. The ferromagnetic coupling is further confirmed by a positive Weiss constant (θ = 1.42 K), determining in the temperature range 3–300 K. By lowering the temperature further, χMT drops down sharply, reaching the minimum value of 1.33 cm3 K mol−1 at 1.8 K. This could be due to zero-field splitting and/or inter-chains antiferromagnetic interaction.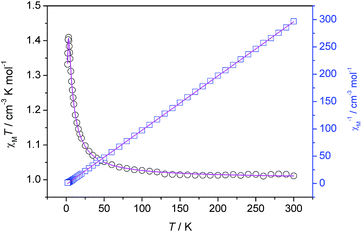 | ||
| Fig. 4 Plot of χMT versus T, 1/χM versus T for 1. The solid line corresponds to the best theoretical fit. | ||
Taking into consideration of structural features of 1 analogous with those of the alternating μ1,1-azido and μ1,3-azido one dimensional coordination polymers, 1 may exhibit alternating ferro- and antiferro-magnetic interaction mediated by bridging PDPH− and μ1,3-azido ligands, respectively.32,33 The magnetic data are fitted as chains using the one-dimensional S = 1/2 expression based on the Hamiltonian
| H = −J1∑S2iS2i+1 − J2∑S2i+1S2i+2 | (1) |
 | (2) |
| u1 = coth(J1S(S + 1)/kT) − kT/J1S(S + 1) |
| u2 = coth(J2S(S + 1)/kT) − kT/J2S(S + 1) |
Considering the weak magnetic interactions between the Cu(II) ions in the adjacent chains, the mean field approximation, zJ′, is introduced. The total magnetic susceptibility is:
 | (3) |
The best-fit parameters are g = 2.09, J1 = 7.36 cm−1, J2 = −0.75 cm−1, zJ′ = −0.017 cm−1. The fitting results show that the magnetic interaction between the Cu(II) ions via PDPH− bridges, and between the Cu(II) ions vis μ1,3-azido ligand are ferromagnetic and antiferromagnetic, respectively. The small negative zJ′ value indicates the magnetic coupling between the Cu(II) ions in the adjacent chains is very weakly antiferromagnetic.
Conclusions
In summary, we have synthesized helical complexes 1–3·OTf by displacements of Cl− in [Cu(PDPH)Cl] by the anion N3−, NO3− and OTf−, respectively. These complexes are isolated and structurally characterised. The results indicated that the counter anions are crucial factors for formation of the different helical structures. μ1,3-azido bridge lead to helical polymer. Weakly coordinating nitrate occupies on coordination site on a Cu center, leading to the double helix structure. No coordinating triflate leaves space for three PDPH− ligands and results in formation of triple helicate. Flexible bispyridylpyrrole backbones accompanying with anions with varied coordination abilities can ligate metal centres in different orientations, and finally give helical structures. Magnetic analyses for 1 reveal that alternating ferro- and antiferromagnetic interactions are operative through the alternating PDPH− and μ1,3-azido bridges, respectively.Acknowledgements
We would like to thank Dr S. S. Bao in Nanjing University and B. Liu in Northwest University for fruitful discussions and valuable suggestions. This work was supported by the National Natural Science Foundation of China (project 21441006), the Open Fund of State Key Laboratory of Medicinal Chemical Biology (Nankai University, 20140513), and the Open-End Fund for the Valuable and Precision Instruments of Central South University.Notes and references
- (a) Y. Sawada, S. Furumi, A. Takai, M. Takeuchi, K. Noguchi and K. Tanaka, J. Am. Chem. Soc., 2012, 134, 4080–4083 CrossRef CAS PubMed; (b) T. Yamamoto, T. Yamada, Y. Nagata and M. Suginome, J. Am. Chem. Soc., 2010, 132, 7899–7901 CrossRef CAS PubMed.
- K. L. Haas and K. Franz, Chem. Rev., 2009, 109, 4921–4960 CrossRef CAS PubMed.
- (a) S. Ye, Y. Wu, W. Zhang, N. Li and B. Tang, Chem. Commun., 2014, 50, 9409–9412 RSC; (b) C. Jobbágy, M. Molnár, P. Baranyai and A. Deák, Dalton Trans., 2014, 43, 11807–11810 RSC.
- M. J. Hannon, V. Moreno, M. J. Prieto, E. Moldrheim, E. Sletten, I. Meistermann, C. J. Isaac, K. J. Sanders and A. Rodger, Angew. Chem., Int. Ed., 2001, 40, 879–884 CrossRef.
- (a) P. Cucos, F. Tuna, L. Sorace, I. Matei, C. Maxim, S. Shova, R. Gheorghe, A. Caneschi, M. Hillebrand and M. Andruh, Inorg. Chem., 2014, 53, 7738–7747 CrossRef CAS PubMed; (b) M. M. Szostak, H. Chojnacki, K. Piela, U. Okwieka-Lupa, E. Bidzińska and K. Dyrek, J. Phys. Chem. A, 2011, 115, 7448–7455 CrossRef CAS PubMed.
- (a) C. Jobbágy, M. Molnár, P. Baranyai, A. Hamza, G. Pálinkás and A. Deák, CrystEngComm, 2014, 16, 3192–3202 RSC; (b) A. El-ghayoury, L. Douce, A. Skoulios and R. Ziessel, Angew. Chem., Int. Ed., 1998, 37, 2205–2208 CrossRef CAS.
- C. J. Matthews, S. T. Onions, G. Morata, L. J. Davis, S. L. Heath and D. J. Price, Angew. Chem., Int. Ed., 2003, 42, 3166–3169 CrossRef CAS PubMed.
- (a) J. Keegan, P. E. Kruger, M. Nieuwenhuyzen and N. Martin, Cryst. Growth Des., 2002, 2, 329–332 CrossRef CAS; (b) J. R. Nitschke, D. Schultz, G. Bernardinelli and D. Gérald, J. Am. Chem. Soc., 2004, 126, 16538–16543 CrossRef CAS PubMed.
- (a) C. J. Baylies, J. C. Jeffery, T. A. Miller, R. Moon, C. R. Rice and T. Riis-Johannessen, Chem. Commun., 2005, 4158–4160 RSC; (b) F. G. Gelalcha, M. Schulz, R. Kluge and J. Sieler, J. Chem. Soc., Dalton Trans., 2002, 2517–2521 RSC.
- (a) C. Piguet, G. Bernardinelli and A. F. Williams, Inorg. Chem., 1989, 28, 2920–2925 CrossRef CAS; (b) K. T. Potts, M. Keshavarz-K, F. S. Tham, H. D. Abruila and C. Arana, Inorg. Chem., 1993, 32, 4450–4456 CrossRef CAS.
- E. C. Constable, S. M. Elder, J. Healy and M. D. Ward, J. Am. Chem. Soc., 1990, 112, 4590–4592 CrossRef CAS.
- D. B. D. Amico, F. Calderazzo, M. Curiardi, L. Labella and F. Marchetti, Inorg. Chem., 2004, 43, 5459–5465 CrossRef PubMed.
- (a) T. Wang and X.-P. Yan, Chem.–Eur. J., 2010, 16, 4639–4649 CrossRef CAS PubMed; (b) D.-X. Wang, S.-X. Fa, Y. Liu, B.-Y. Hou and M.-X. Wang, Chem. Commun., 2012, 48, 11458–11460 RSC.
- (a) W. Feng, Y. Zhang, Z. Zhang, X. Lü, H. Liu, G. Shi, D. Zou, J. Song, D. Fan, W.-K. Wong and R. A. Jones, Inorg. Chem., 2012, 51, 11377–11386 CrossRef CAS PubMed; (b) M. Hirotsu, Y. Shimizu, N. Kuwamura, R. Tanaka, I. Kinoshita, R. Takada, Y. Teki and H. Hashimoto, Inorg. Chem., 2012, 51, 766–768 CrossRef CAS PubMed.
- (a) Y.-Q. Huang, X.-Q. Zhao, W. Shi, W.-Y. Liu, Z.-L. Chen, P. Cheng, D.-Z. Liao and S.-P. Yan, Cryst. Growth Des., 2008, 8, 3652–3660 CrossRef CAS; (b) T. F. Mastropietro, N. Marino, D. Armentano, G. D. Munno, C. Yuste, F. Lloret and M. Julve, Cryst. Growth Des., 2013, 13, 270–281 CrossRef CAS.
- (a) H.-J. Kim, W.-C. Zin and M. Lee, J. Am. Chem. Soc., 2004, 126, 7009–7014 CrossRef CAS PubMed; (b) J. Chen, S.-H. Wang, Z.-F. Liu, M.-F. Wu, Y. Xiao, F.-K. Zheng, G.-C. Guo and J.-S. Huang, New J. Chem., 2014, 38, 269–276 RSC; (c) T. F. Mastropietro, N. Marino, D. Armentano, G. D. Munno, C. Yuste, F. Lloret and M. Julve, Cryst. Growth Des., 2013, 13, 270–281 CrossRef CAS.
- B. Hasenknopf, J.-M. Lehn, N. Boumediene, A. Dupont-Gervais, A. Van Dorsselaer, B. Kneisel and D. Fenske, J. Am. Chem. Soc., 1997, 119, 10956–10962 CrossRef CAS.
- B. Hasenknopf, J.-M. Lehn, B. O. Kneisel, G. Baum and D. Fenske, Angew. Chem., Int. Ed. Engl., 1996, 35, 1838–1840 CrossRef CAS.
- M. Albrecht, Chem. Rev., 2001, 101, 3457–3498 CrossRef CAS PubMed.
- W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688–764 CrossRef CAS PubMed.
- J. W. Ciszek, Z. K. Keane, L. Cheng, M. P. Stewart, L. H. Yu, D. Natelson and J. M. Tour, J. Am. Chem. Soc., 2006, 128, 3179–3189 CrossRef CAS PubMed.
- G. H. Imler, Z. Lu, K. A. Kistler, P. J. Carroll, B. B. Wayland and M. J. Zdilla, Inorg. Chem., 2012, 51, 10122–10128 CrossRef CAS PubMed.
- F. Hein and U. Beierlein, Pharm. Zentralhalle Dtschl., 1957, 96, 401–421 CAS.
- X. H. Hu, Y. Liang, C. Li and X. Y. Yi, Dalton Trans., 2014, 43, 2458–2464 RSC.
- (a) H. Schindlbauer, Monatsh. Chem., 1968, 99, 1799–1807 CrossRef CAS; (b) R. A. Jones, M. Karatza, T. N. Voro, P. U. Civcir, A. Franck, O. Ozturk, J. P. Seaman, A. P. Whitmore and D. J. Williamson, Tetrahedron, 1996, 52, 8707–8724 CrossRef CAS.
- M. Rui, X. H. Hu, X. Y. Yi and S. C. Zhang, J. Cent. South Univ., 2014 Search PubMed , accepted.
- G. M. Sheldrick, SADABS, University of Göttingen, Germany, 1997 Search PubMed.
- G. M. Sheldrick, SHELXTL-Plus V5.1 Software Reference Manual, Bruker AXS Inc., Madison, Wisconsin, USA, 1997 Search PubMed.
- A. Messimeri, C. Papadimitriou, C. P. Raptopoulou, A. Escuer, S. P. Perlepes and A. K. Boudalis, Inorg. Chem. Commun., 2007, 10, 800–804 CrossRef CAS PubMed.
- (a) Y. Cui and C. He, J. Am. Chem. Soc., 2003, 125, 16202–16203 CrossRef CAS PubMed; (b) G. Baum, E. C. Constable, D. Fenske, C. E. Housecroft and T. Kulke, Chem. Commun., 1998, 2659–2660 Search PubMed; (c) E. C. Constable, T. Kulke, M. Neuburger and M. Zehnder, Chem. Commun., 1997, 489–490 RSC.
- (a) Effendy, F. Marchetti, C. Pettinari, R. Pettinari, B. W. Skelton and A. H. White, Inorg. Chim. Acta, 2007, 360, 1414–1423 CrossRef CAS PubMed; (b) H. Feng, X.-P. Zhou, T. Wu, D. Li, Y.-G. Yin and S. W. Ng, Inorg. Chim. Acta, 2006, 359, 4027–4035 CrossRef CAS PubMed; (c) Z. Ma, Y.-P. Xing, M. Yang, M. Hu, B.-Q. Liu, M. F. C. G. da Silva and A. J. L. Pombeiro, Inorg. Chim. Acta, 2009, 362, 2921–2926 CrossRef CAS PubMed; (d) M. J. Hannon, C. L. Painting, E. A. Plummer, L. J. Childs and N. W. Alcock, Chem.–Eur. J., 2002, 8, 2225–2238 CrossRef CAS.
- E. Q. Ago, S. Q. Bai, Y. F. Yue, Z. M. Wang and C. H. Yan, Inorg. Chem., 2003, 42, 3642–3649 CrossRef PubMed.
- R. Cortés, M. Drillon, X. Solans, L. Lezama and T. Rojo, Inorg. Chem., 1997, 36, 677–683 CrossRef.
Footnote |
| † CCDC 1015413–1015415. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra15191g |
| This journal is © The Royal Society of Chemistry 2015 |