DOI:
10.1039/C4RA12047G
(Paper)
RSC Adv., 2015,
5, 8739-8752
Curcumin-p-sulfonatocalix[4]resorcinarene (p-SC[4]R) interaction: thermo-physico chemistry, stability and biological evaluation†
Received
9th October 2014
, Accepted 8th December 2014
First published on 11th December 2014
Abstract
The new 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex formation of curcumin–p-SC[4]R has been investigated with the aim to enhance the solubility, bioavailability, stability and anti-oxidant activity as well as decrease in vivo acute oral toxicity of curcumin by inclusion complexation. Thermodynamic parameters ΔS and ΔH are negative, indicating that the inclusion complexation was an exothermic process, which occurred spontaneously. The inclusion complex was characterised by different analytical methods, including FT-IR, PXRD, 1H-NMR, SEM, DSC, ESI-mass, UV-Vis spectroscopy and elemental analysis.
1 stoichiometric complex formation of curcumin–p-SC[4]R has been investigated with the aim to enhance the solubility, bioavailability, stability and anti-oxidant activity as well as decrease in vivo acute oral toxicity of curcumin by inclusion complexation. Thermodynamic parameters ΔS and ΔH are negative, indicating that the inclusion complexation was an exothermic process, which occurred spontaneously. The inclusion complex was characterised by different analytical methods, including FT-IR, PXRD, 1H-NMR, SEM, DSC, ESI-mass, UV-Vis spectroscopy and elemental analysis.
Introduction
Curcumin (CUR), bis (4-hydroxy-3-methoxyphenyl)-1,6-diene-3,5-dione, is a natural, low molecular weight, hydrophobic yellow-orange polyphenolic compound that is isolated from the rhizome of the spice herb curcuma longa Linn., commonly known as turmeric, belonging to the ginger family (Zingiberaceae). Turmeric has been extensively cultivated in India and other Asian countries for its use in cooking, textiles, as cosmetic agent for skin care, and therapeutic uses. It is approved as a food additive by the WHO and the Food & Agriculture Organization.1
Research over the past decade has shown that curcumin has been extensively investigated for its potential therapeutic benefits with nominal side effects.2 The high pharmacological efficacy and safety profile of curcumin makes it a potential compound for the treatment and prevention of a wide variety of human diseases. However, in spite of the applicable qualities and biological activity of this bioactive molecule, it has not yet been approved as a therapeutic agent because of its poor solubility, low bioavailability and stability (short half-life).3 The inferior bioavailability of curcumin is due to its low absorption, rapid metabolism and systematic elimination from the biological system.4 Thus, an enhancement in the solubility, stability and bioavailability of curcumin is essential.
Many researchers are involved in the development of new strategies to improve the solubility, stability and bioavailability of curcumin and have reported several methods.5–9 However, these methods have disadvantages such as the change in the activity of modified curcumin and dispersion of nanoparticles.10 Therefore, T. Harada and co-workers have examined the encapsulation of curcumin with cyclodextrin,11 However, cyclodextrin is present inside the cell and it leads to deformation of the cell structure.12 Fascinatingly, recent studies show that cell transfection of calixarenes does not affect the structure of the cell and has a number of medicinal applications.13 Recently, using the encapsulation of curcumin with para-sulfonatocalix[4]arene, an attempt has been made to increase the solubility and bioavailability of curcumin, which was investigated by UV and fluorescence spectroscopic methods.14 However, no such study has been reported with p-SC[4]R and hence the present study was designed.
Calixarenes are one of the major classes of macro cyclic organic compounds in supramolecular chemistry along with cyclodextrins, crown-ethers, cryptands and curcurbiturils, which are described as macro cycles having almost unlimited possibilities due to their ease of modification on lower as well as upper rims.15 Calixresorcinarenes, a member of the calixarenes, are large cyclic tetramers obtained from the acid-catalysed condensation of resorcinol with appropriate aldehyde. One of the characteristics of calixarenes is their insolubility in water, therefore Shinkai and co-workers have reported water soluble calixarene derivatives bearing sulfonic acid groups at the upper rims.16 This water soluble modified calixarenes can be a good medium to study inclusion behaviour. Moreover, sulfonatocalixarenes have become a particularly important class in host–guest supramolecular chemistry because of their high solubility in water, stability and lower toxicity than cyclodextrins with a number of potential biological activities.17
Calixresorcinarenes are third generation macro cyclic host molecules composed of resorcinol units linked by methylene bridges at 2- and 6-position.18 The bowl shaped structure of calixresorcinarenes have a concave binding cavity and high affinity towards various guests such as cations, anions and molecules with different sizes and various hydrophobic/hydrophilic characteristics.19–21 The formation of inclusion complexes with biological compounds is an interesting application of the functionalized calixarenes. The development of water soluble calixarenes as receptors for biomolecules has become a very important field of research work in view of their potential applications to increase the bioavailability and to decrease the systemic toxicity of the biologically active compounds.22 Recently, there has been a report from our laboratory on the binding studies of p-SC[4]R with the biologically important drug molecule lamotrigine.23
The aim of the present study is to explore the effect of water soluble p-SC[4]R on the dissolution behaviour of curcumin. We selected p-SC[4]R as a drug solubilizing agent due to the ease of synthesis, availability of 8 phenolic units and simple accessibility. In addition, they possess an open and rigid structure and also a number of possible conformations and binding positions with a hydrophilic outer surface and an apolar cavity at their centre that provides a hydrophobic matrix. Because curcumin is a symmetric lipophilic molecule, it can be easily entrapped in the hydrophobic p-SC[4]R cavity to form a host–guest complex. We have carried out investigations, including UV-Vis spectroscopy, FT-IR spectroscopy, powder X-ray diffractometry, 1H-NMR, SEM, DSC and ESI-mass spectroscopy of the host–guest interactions between p-SC[4]R and curcumin. The stoichiometry of the inclusion complex and the apparent formation constant have been estimated. We have performed studies on the phase solubility, in vivo toxicity as well as the stability of the inclusion complex formed and also determined the anti-oxidant ability by the DPPH radical scavenging activity of the inclusion complex.
Experimental section
Animals
The Swiss albino mice (Mus musculus) were procured from the Laboratory Animal Centre of Zydus Research Center (ZRC), Ahmedabad, under the Animal Maintenance and License no. 167/1999/CPCSEA from the Ministry of Social Justice and Empowerment, Government of India. The animal care, handling and the protocols were approved by the Institutional Animal Ethics Committee (IAEC), India. The animals were acclimatized under a well regulated 12 h light–dark schedule at 26 ± 2 °C and relative humidity of 45–60% for 1 week before experiments.
Chemicals and reagents
Curcumin (MW, 368.38; purity ≥ 99.0%), resorcinol (purity ≥ 99.8%), DPPH and other chemicals were purchased from Sigma Aldrich. All other reagents were analytical grade reagents and were used without purification. The host p-SC[4]R (MW, 864.84) was synthesized according to the previously reported procedure.18,23 The p-SC[4]R was characterized by various spectroscopic techniques and the data were compared with standard values. Ultra-pure water from a Millipore Synergy system (Millipore, Bedford, MA, USA) was used for the preparation of inclusion complexes.
Instruments
UV-Vis measurements were performed for curcumin, p-SC[4]R and the inclusion complex by a Jasco V-570 spectrophotometer at ambient temperature (25 ± 1 °C). All the samples were dissolved in a H2O–EtOH (50%) mixture and scanned in the 200 to 600 nm wavelength range to obtain the absorption spectra. The FT-IR spectra were obtained using a Bruker Tensor-27 FT-IR spectrometer with KBr pellets in the range of 4000–400 cm−1. Elemental analyses were performed using GmbH Vario Micro cube elemental analyzer. 1H-NMR spectra were recorded using a Bruker Avance III 400 spectrophotometer at 400 MHz, 500 MHz and 125 MHz with TMS as an internal standard. The following abbreviations were used to indicate NMR-multiplicities: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad). The ESI-MS experiments of all samples were performed using an Applied Bio systems, API 2000 LC/MS/MS instrument (USA) equipped with an electrospray ionization source operating in the positive ion mode. X-ray diffraction (XRD) patterns were performed using a SEIFERT-FPM (XRD7), using Cu Kα X-ray lines at 1.5406 Å as the radiation source at 40 kV and 30 mA power and also Make Philips X'PERT MPD. The liquid chromatographic system used was an isocratic HPLC Waters system (USA) consisting of a Waters 515 HPLC pump, Waters variable wavelength UV-Vis detector equipped with Waters Empower 2 solution software and a sample injector fitted with a 20 μL sample loop using a C18 system column (150 × 3.9 mm I.D., 5 μm particle size, Waters, USA) at ambient temperature (25 ± 1 °C). The detection wavelength was set at 428 nm. The mobile phase was a mixture of methanol–H2O (50%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50%, v/v) (containing 3% glacial acetic acid) filtered with 0.45 μm membrane filter paper at 1.0 mL min−1 flow rate. DSC analyses of all samples were performed in a Shimadzu model DSC-60 (Japan) calibrated with indium. SEM microscopy of curcumin, p-SC[4]R, a physical mixture of curcumin–p-SC[4]R and the inclusion complex were performed with a Leo 440i, Leo Electron Microscopy Ltd, Cambridge CB1 3QH, England, to visualize the surface morphology.
50%, v/v) (containing 3% glacial acetic acid) filtered with 0.45 μm membrane filter paper at 1.0 mL min−1 flow rate. DSC analyses of all samples were performed in a Shimadzu model DSC-60 (Japan) calibrated with indium. SEM microscopy of curcumin, p-SC[4]R, a physical mixture of curcumin–p-SC[4]R and the inclusion complex were performed with a Leo 440i, Leo Electron Microscopy Ltd, Cambridge CB1 3QH, England, to visualize the surface morphology.
Preparation of the inclusion complex of curcumin and p-SC[4]R
The inclusion complex was prepared by mixing curcumin and p-SC[4]R according to the previously reported method described by Menon.23 Briefly, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of curcumin (0.368 g, 1 mM) and p-SC[4]R (0.865 g, 1 mM) were dissolved in 100 mL of ultrapure water, stirred for 48 h at 37 °C by rotary shaker in the dark and filtered through 0.45 μm membrane filter to eliminate undissolved materials. The filtrate was dried at 60 °C under 2–5 torr vacuum to collect the inclusion complex of curcumin and p-SC[4]R (Scheme 1).
1 molar ratio of curcumin (0.368 g, 1 mM) and p-SC[4]R (0.865 g, 1 mM) were dissolved in 100 mL of ultrapure water, stirred for 48 h at 37 °C by rotary shaker in the dark and filtered through 0.45 μm membrane filter to eliminate undissolved materials. The filtrate was dried at 60 °C under 2–5 torr vacuum to collect the inclusion complex of curcumin and p-SC[4]R (Scheme 1).
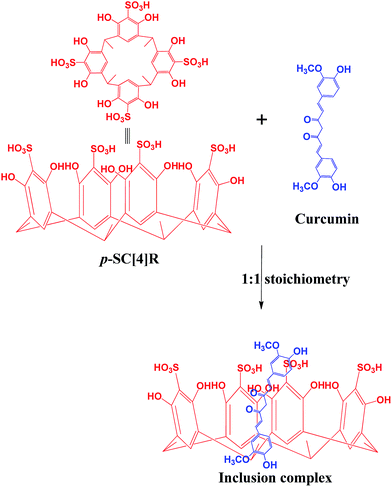 |
| | Scheme 1 Proposed mechanism of curcumin–p-SC[4]R inclusion complex [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]. 1]. | |
Preparation of the physical mixture of curcumin and p-SC[4]R
A physical mixture of curcumin–p-SC[4]R having a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio was prepared by simple grinding using an agate mortar and pestle to obtain a uniform physical mixture.
1 molar ratio was prepared by simple grinding using an agate mortar and pestle to obtain a uniform physical mixture.
In vitro evaluations of curcumin–p-SC[4]R inclusion complex
Powder dissolution studies. Dissolution studies of pure curcumin, the physical mixture of curcumin–p-SC[4]R and the inclusion complex were conducted as per the standard protocol using USP rotating paddle type-II apparatus (USP-II) filled with 900 mL of 0.2 M HCl aqueous solution of dissolution medium (pH 1.2), maintained at 37 ± 1 °C and agitation speed at 100 rpm.23 All samples containing 30 mg of curcumin were used and sprinkled directly onto the surface of the dissolution medium. Aliquots of dissolution samples were withdrawn at predetermined intervals, filtered through a 0.45 μm membrane filter and analyzed by HPLC with a UV detector. An equivalent amount of fresh dissolution medium was added to maintain constant volume and sink conditions. The dissolution experiments were conducted in triplicate at 37 °C.
Phase solubility analysis. The phase solubility analysis of curcumin with p-SC[4]R was conducted according to the method described by Higuchi and Connors with some minor modifications.24 In brief, an excess amount of curcumin was added to each 10 mL sample of p-SC[4]R aqueous solution with different concentrations ranging from 0 to 0.0012 mM. Each flask was capped and sonicated for 1 h and agitated for 72 h at 25 ± 1 °C in an orbit shaker incubator (Newtronics, India) at 100 rpm. After attaining equilibrium, the suspensions were filtered through 0.45 μm membrane filters to remove undissolved compounds. The resulting clear filtrate was diluted and analysed by HPLC. All experiments were done in triplicate and the samples were protected from light. The phase solubility diagram was constructed by plotting the concentration of curcumin against the concentration of the p-SC[4]R. In the case of the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex, the apparent stability constants, Kc, were calculated from the slope and intercept, So, of the initial straight line portion of the phase solubility diagrams using eqn (1) given as follows:
1 inclusion complex, the apparent stability constants, Kc, were calculated from the slope and intercept, So, of the initial straight line portion of the phase solubility diagrams using eqn (1) given as follows:| |
 | (1) |
where, So is the intrinsic solubility of curcumin in the absence of p-SC[4]R and slope is the corresponding slope of the phase solubility diagram.
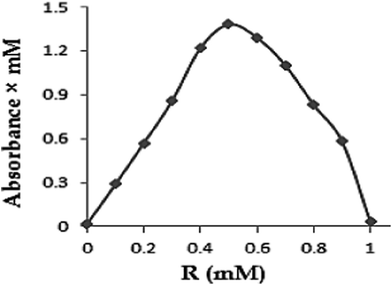
Determination of Job's plot. Job's method involves the determination of the binding stoichiometry of curcumin and p-SC[4]R in aqueous solution according to the reported method with minor variation.25 Briefly, equimolar (0.01 mM) EtOH–water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; v/v) solutions of curcumin and p-SC[4]R were mixed to a fixed volume by varying the molar ratio from 0.1 to 1, keeping the total concentration of solutes constant. After 1 h of stirring, the absorbance of each solution was measured by UV-Vis spectroscopy at 428 nm. The difference between the absorbance of curcumin with and without p-SC[4]R was plotted against R, where R is obtained by eqn (2) given as follows:
50; v/v) solutions of curcumin and p-SC[4]R were mixed to a fixed volume by varying the molar ratio from 0.1 to 1, keeping the total concentration of solutes constant. After 1 h of stirring, the absorbance of each solution was measured by UV-Vis spectroscopy at 428 nm. The difference between the absorbance of curcumin with and without p-SC[4]R was plotted against R, where R is obtained by eqn (2) given as follows:| |
 | (2) |
Effect of temperature on the curcumin–p-SC[4]R complexation
The effect of temperature on the solubility of curcumin with p-SC[4]R was determined similar to the phase solubility study described above; the only variation was in the temperature of the medium. The complexation study was investigated at different temperatures of 25, 35 and 45 °C. The phase solubility diagrams were plotted as defined above and the thermodynamic parameters of the inclusion complex between curcumin and p-SC[4]R were obtained by plotting the logarithm of the stability constants against the correlative of temperature. The values of enthalpy and entropy deviations were calculated following the Van't Hoff eqn (3), and free energy changes for the complexation were calculated by the Gibbs eqn (4).| |
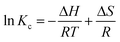 | (3) |
| |
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc
| (4) |
Stability studies of curcumin–p-SC[4]R complex
Storage stability. The colorant curcumin and the inclusion complex of curcumin–p-SC[4]R solutions were prepared by dissolving 1 g each in 100 mL of Milli-Q water and dividing into four groups at room temperature (∼25 °C). One group was placed in the dark, another group was placed in natural light, a third group was placed in an incubator set at 45 °C temperature to investigate the effect of heat, and the last group was exposed to UV light to investigate the effect of UV radiation on the stability of curcumin for 40 days. Quantitative analyses were performed at weekly intervals and the concentration of the appropriate dilutions of curcumin were analyzed by spectrophotometry.8,26 The results were expressed as percentages of the remaining curcumin. The colorant concentration was monitored during the experimental period and the calculated degradation rate constant was analyzed by linear regression analysis of the logarithm of the percentages of remaining curcumin against time. Each sample analysis was repeated twice and the mean value was considered.
pH stability. Pure and inclusion complexed curcumin, 0.001 mM of colorants were diluted in a water–ethanol solution in a ratio of 50%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% (v/v). The solutions were prepared just before taking the measurements. The 5 mL solutions, were adjusted to pH values in the range of 2–12 using buffer solutions27 and the wavelength of absorbance was determined at 428 nm by a Jasco V-570 spectrophotometer.
50% (v/v). The solutions were prepared just before taking the measurements. The 5 mL solutions, were adjusted to pH values in the range of 2–12 using buffer solutions27 and the wavelength of absorbance was determined at 428 nm by a Jasco V-570 spectrophotometer.
Assay of anti-oxidant activities. The anti-oxidant activity of the inclusion complex of curcumin–p-SC[4]R was compared to curcumin by a DPPH radical scavenging assay method.26 In brief, a series of concentrations (1 mg mL−1) of pure curcumin, p-SC[4]R and its inclusion complex were dissolved in methanol–water (50%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50%; v/v) and appropriately diluted. A 0.3 mM (0.013 g mL−1) of DPPH solution was prepared in methanol–water (50%
50%; v/v) and appropriately diluted. A 0.3 mM (0.013 g mL−1) of DPPH solution was prepared in methanol–water (50%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50%; v/v). Then, 2 mL of DPPH solution were added to 2 mL of sample solutions at different concentration and the mixtures were incubated at room temperature for 30 min. The free radical scavenging activity of the inclusion complex as well as pure curcumin and p-SC[4]R were measured by monitoring the decay of absorbance of the DPPH solution at 518 nm in the presence of the inclusion complex as well as p-SC[4]R and curcumin alone using a Jasco V-570 UV-Vis spectrophotometer. The anti-oxidant activity of the solutions as well as that of ascorbic acid was assayed by the same procedure for comparison. The experiment was performed in triplicate. DPPH scavenging activity was calculated as per the eqn (5) given as follows:
50%; v/v). Then, 2 mL of DPPH solution were added to 2 mL of sample solutions at different concentration and the mixtures were incubated at room temperature for 30 min. The free radical scavenging activity of the inclusion complex as well as pure curcumin and p-SC[4]R were measured by monitoring the decay of absorbance of the DPPH solution at 518 nm in the presence of the inclusion complex as well as p-SC[4]R and curcumin alone using a Jasco V-570 UV-Vis spectrophotometer. The anti-oxidant activity of the solutions as well as that of ascorbic acid was assayed by the same procedure for comparison. The experiment was performed in triplicate. DPPH scavenging activity was calculated as per the eqn (5) given as follows:| |
 | (5) |
where Ac is the absorbance of the DPPH solution and As is the absorbance of the DPPH solution with the inclusion complex as well as pure curcumin and p-SC[4]R.
In vivo evaluations of the curcumin–p-SC[4]R complexation
Acute oral toxicity (LD50). The acute oral toxicity of the inclusion complex of curcumin–p-SC[4]R was evaluated in Swiss albino mice using the Organization for Economic Co-operation and Development (OECD) guidelines 425 (OECD, 3 October, 2008) (Acute Oral Toxicity-Modified Up and Down Procedure). The procedure followed was according to the earlier reported method described by Menon et al.23 Swiss albino mice were used to assess the toxicity level. The mice were fasted overnight prior to the dosing. During the period of fasting, mice were weighed and the inclusion complex of curcumin with p-SC[4]R were administered. The inclusion complex of curcumin with p-SC[4]R at a dose of 2750 mg kg−1 was administered to four Swiss albino mice in a single dose by oral gavage. All mice were observed individually after dosing at least once during the first 30 min, and periodically during the first 24 h with special attention being given during the first 4 h and daily thereafter, for a total of 14 days, for clinical signs as well as for morbidity and mortality. The fasted body weight of each mice is determined and the dose is calculated according to the body weight. The acute oral toxicity studies were calculated by the AOT425 software prepared by US Environmental Protection Agency.
Statistical analysis. All of the experiments in the study were performed using the origin 6.1 software. All the samples were prepared and analyzed in triplicate. The data were expressed as the mean ± standard deviation (SD). The differences were detected by one-way analysis of variance (ANOVA) with a statistical significance for p value <0.05.
Results and discussion
In this study, we prepared the solid inclusion complex of curcumin–p-SC[4]R, which was confirmed by various spectroscopic and physicochemical methods. We also investigated in vitro as well as in vivo assessments, which are discussed below.
Characterization of the solid complex of curcumin–p-SC[4]R
Yield 0.77 g, (89%), elemental analysis for C53H52O26S4 (1232.16) C, 51.62%; H, 4.25%; O, 33.73%; S, 10.40%, found C, 51.65%; H, 4.23%; O, 33.75%; S, 10.37%. FT-IR (KBr) ν, cm−1 3510 (–OH stretching, broadened), 1629 (strong –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1605 (–C
O), 1605 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration), 1459 (–C–O), 1191 (–SO3H), 1026 (–C–O–C– stretching). 1H NMR (400 MHz, DMSO-d6): δ = 3.813 (S, 6H, J = 11.5 Hz, –OCH3), 4.523 (S, 2H, J = 12.40, –CH methylidine), 4.104 (m, 4H, J = 6.4 Hz, –CH), 6.627 (d, 2H, –CH), 6.751 (d, 2H, –CH), 6.784 (S, 2H, –CH), 6.883 (S, 2H, –CH), 7.090 (S, 4H, Ar-H), 7.431 (d, 2H, –CH), 7.562 (S, 2H, –OH), 9.891 (S, 4H, –SO3H), 8.451 (S, 8H, –OH, p-SC[4]R), 1.247 (d, 12H, J = 6.8 Hz, –CH3). ESI-MS m/z 1232.1 (Curcumin + p-SC[4]R).
C stretching vibration), 1459 (–C–O), 1191 (–SO3H), 1026 (–C–O–C– stretching). 1H NMR (400 MHz, DMSO-d6): δ = 3.813 (S, 6H, J = 11.5 Hz, –OCH3), 4.523 (S, 2H, J = 12.40, –CH methylidine), 4.104 (m, 4H, J = 6.4 Hz, –CH), 6.627 (d, 2H, –CH), 6.751 (d, 2H, –CH), 6.784 (S, 2H, –CH), 6.883 (S, 2H, –CH), 7.090 (S, 4H, Ar-H), 7.431 (d, 2H, –CH), 7.562 (S, 2H, –OH), 9.891 (S, 4H, –SO3H), 8.451 (S, 8H, –OH, p-SC[4]R), 1.247 (d, 12H, J = 6.8 Hz, –CH3). ESI-MS m/z 1232.1 (Curcumin + p-SC[4]R).
UV-Vis analysis
The UV-Vis spectra of curcumin, p-SC[4]R and the inclusion complex of curcumin–p-SC[4]R with visual colour changes are shown in Fig. 1. The results show three peaks at 428, 458 and 585 nm for pure curcumin, p-SC[4]R and the inclusion complex, respectively. The wavelength of maximum absorption (λmax) of curcumin was remarkably altered by the addition of p-SC[4]R. Apparent absorption changes of curcumin have been observed with and without p-SC[4]R. On addition of p-SC[4]R, the absorption maximum of free curcumin showed a considerable redshift from 428 nm to 585 nm with a concomitant increase in the absorption intensity. Thus, the UV-Vis spectra indicate the successful formation of the inclusion complex between curcumin and p-SC[4]R.
 |
| | Fig. 1 UV-Vis absorption spectra of pure curcumin, p-SC[4]R and the inclusion complex in H2O–EtOH (50%) with visual colour changes. | |
FT-IR analysis
The FT-IR spectra of curcumin, p-SC[4]R, the physical mixture of curcumin–p-SC[4]R and the inclusion complex are presented in Fig. 2. The FT-IR spectrum of pure curcumin shows a sharp absorption band at 3510 cm−1, indicating the presence of the phenolic –OH stretching vibration. The strong peak at 1628 cm−1 represent predominantly mixed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups, and 1603 cm−1 is indicative of the symmetric aromatic stretching vibration. The sharp peak at 1455 cm−1 is attributed to the phenolic C–O, whereas the enolic C–O peak was obtained at 1275 cm−1. Absorption at 1025 cm−1 is indicative of the C–O–C asymmetric stretching vibration. The peak at 958 cm−1 is due to the benzoate trans C–H vibration and at 712 cm−1 is the C–H vibration of the aromatic ring. The FT-IR spectrum of p-SC[4]R consists of broad absorption bands of –OH stretching at 3456 cm−1, whereas three characteristic absorption peaks at 1187, 1115 and 1057 cm−1 are indicative of the –SO3 group. The FT-IR spectra of the physical mixture is found to have merged with lower intense broad OH peak due to the interactions with supramolecule during complex formation, and moreover, all the sharp peaks of curcumin as well as p-SC[4]R are observed along with a few other peaks and reveal reduced sharpness indicating that the spectrum of the physical mixture was essentially a combination of the spectra of the two molecules of curcumin with p-SC[4]R. However, the curcumin–p-SC[4]R inclusion complex spectrum shows no features similar to that of pure curcumin. Several insignificant absorption peaks of curcumin from 500–1200 cm−1 almost disappeared and the sharp –OH stretching band of curcumin at 3510 cm−1 broadened, which indicates the inclusion of curcumin into the p-SC[4]R cavity.
C groups, and 1603 cm−1 is indicative of the symmetric aromatic stretching vibration. The sharp peak at 1455 cm−1 is attributed to the phenolic C–O, whereas the enolic C–O peak was obtained at 1275 cm−1. Absorption at 1025 cm−1 is indicative of the C–O–C asymmetric stretching vibration. The peak at 958 cm−1 is due to the benzoate trans C–H vibration and at 712 cm−1 is the C–H vibration of the aromatic ring. The FT-IR spectrum of p-SC[4]R consists of broad absorption bands of –OH stretching at 3456 cm−1, whereas three characteristic absorption peaks at 1187, 1115 and 1057 cm−1 are indicative of the –SO3 group. The FT-IR spectra of the physical mixture is found to have merged with lower intense broad OH peak due to the interactions with supramolecule during complex formation, and moreover, all the sharp peaks of curcumin as well as p-SC[4]R are observed along with a few other peaks and reveal reduced sharpness indicating that the spectrum of the physical mixture was essentially a combination of the spectra of the two molecules of curcumin with p-SC[4]R. However, the curcumin–p-SC[4]R inclusion complex spectrum shows no features similar to that of pure curcumin. Several insignificant absorption peaks of curcumin from 500–1200 cm−1 almost disappeared and the sharp –OH stretching band of curcumin at 3510 cm−1 broadened, which indicates the inclusion of curcumin into the p-SC[4]R cavity.
 |
| | Fig. 2 FT-IR spectra of (A) curcumin, (B) p-SC[4]R, (C) physical mixture and (D) inclusion complex. | |
PXRD analysis
Powder X-ray diffractometry is a useful method, which has been proven to provide insightful information for studying the complexation between p-SC[4]R and curcumin. Fig. 3 shows the XRD pattern of curcumin, p-SC[4]R, the physical mixture of curcumin–p-SC[4]R and the inclusion complex. The XRD pattern of curcumin shows intense and sharp peaks, indicating its crystalline nature. The XRD pattern of curcumin has major diffraction peaks at 2θ ≅ 12.14°, 14.84°, 17.67°, 18.75°, 21.72°, 24.09°, 25.22° and 27.77°, whereas the XRD pattern of p-SC[4]R reveals three broad peaks at 2θ ≅ 11.08°, 18.61° and 23.22°, consistent with its amorphous character. The XRD pattern of the physical mixture displays essentially a superposition of the patterns of curcumin and p-SC[4]R, confirming the absence of complex formation, and both retained their original physical characteristics. However, the XRD pattern of as received curcumin with p-SC[4]R inclusion complex shows no diffraction peaks except a halo diffraction pattern, indicating that the material is typically amorphous, having some amount of channel type structure and exhibits none of the characteristic peaks of curcumin indicating that complete complexation was achieved between curcumin and p-SC[4]R.
 |
| | Fig. 3 Powder X-ray diffraction patterns of curcumin, p-SC[4]R, physical mixture and inclusion complex. | |
HPLC analysis
The results of HPLC analysis of pure curcumin, p-SC[4]R and the inclusion complex of curcumin with p-SC[4]R, using the isocratic reverse phase HPLC method, are shown in Fig. S1 (ESI†). The chromatograms of pure curcumin and p-SC[4]R show peaks at retention times of 6.75 min and 12.90 min, respectively. The chromatogram of the inclusion complex also shows the peaks of both curcumin and p-SC[4]R at the same retention times, indicating the existence of both curcumin as well as p-SC[4]R in the inclusion complex.
1H-NMR analysis
NMR spectroscopy is widely used and indeed the most powerful technique for the study of the inclusion of the guest curcumin into the hydrophobic p-SC[4]R cavity in solution. The inclusion complex of curcumin in the p-SC[4]R cavity is confirmed by the change in chemical shifts of the guest and host protons, in comparison with the chemical shifts of the protons in the free molecules. The 1H-NMR spectra of pure curcumin, p-SC[4]R and the curcumin–p-SC[4]R inclusion complex are shown in Fig. 4 with DMSO-d6 as a solvent. The chemical shift (δ) values of curcumin, p-SC[4]R and the inclusion complex are given in Table 1.
 |
| | Fig. 4 1H-NMR spectrum of curcumin, p-SC[4]R and curcumin–p-SC[4]R inclusion complex in DMSO-d6. | |
Table 1 Chemical shift values (δ) of the 1H NMR spectra corresponding to curcumin in the presence and absence of p-SC[4]R in DMSO-d6
| Proton assignment |
δ0 curcumin (ppm) |
δ0 p-SC[4]R (ppm) |
δ curcumin–p-SC[4]R complex (ppm) |
Δδ = δ − δ0 (ppm) |
| a |
3.819, 3.838 |
— |
3.813 |
−0.006, −0.025 |
| b |
4.528 |
— |
4.523 |
−0.005 |
| c |
7.567, 7.589 |
— |
7.562 |
−0.005, −0.027 |
| d |
— |
9.885 |
9.891 |
0.006 |
| e |
— |
8.439 |
8.451 |
0.012 |
| f |
— |
1.243 |
1.247 |
0.004 |
Generally, it was observed that the aromatic part of curcumin and methylene bridged protons (a, b & c-protons) were shifted to the up field (lower chemical shift in ppm) region in the complex as compared to pure curcumin protons. Neither the appearance of new peaks nor splitting was observed in this complex. These chemical shifts resulted owing to the binding of the aromatic segment of curcumin within the hydrophobic cavity of p-SC[4]R, and the ring current effect resulted in the shielding of protons. On the other hand, the peaks (d, e & f-protons) corresponding to p-SC[4]R are shifted to the downfield (higher chemical shift in ppm) region due to the binding of curcumin with p-SC[4]R inside the cavity via π–π interactions, as well as the wide rim with stronger interactions with its hydroxyl groups. This data indicates that the differences in chemical shifts (ppm) for curcumin peaks in the curcumin–p-SC[4]R complex compared to pure curcumin are due to the presence of strong complexation. Thus the 1H-NMR spectra strongly confirm that the guest molecule curcumin penetrates deeply into the hydrophobic cavity of p-SC[4]R.
DSC analysis
Fig. 5 shows the DSC curves of pure curcumin, p-SC[4]R, their physical mixture and the inclusion complex of curcumin with p-SC[4]R. The DSC curves of curcumin present a single sharp endothermic peak at 183 °C, corresponding to the melting point of crystalline curcumin, followed by an exothermic influence because of the thermal degradation of the substance at higher temperatures. The DSC thermogram of p-SC[4]R displays a relatively broad peak appearing at 235 °C, which could have been formed owing to its amorphous nature. The DSC thermogram of the physical mixture exhibits a combination of the curcumin as well as p-SC[4]R molecules indicating the absence of close association between these molecules. Moreover, the variance of the curcumin thermogram intensity is reduced, whereas the two molecules are simply mixed together. In contrast, the DSC thermogram of the inclusion complex of curcumin with p-SC[4]R shows the disappearance of the melting endothermic peak of curcumin. The appearance of a new small peak at 174 °C together with the shifting of one representative band is suggestive of a change in the structure and of a compact interaction between curcumin and p-SC[4]R.
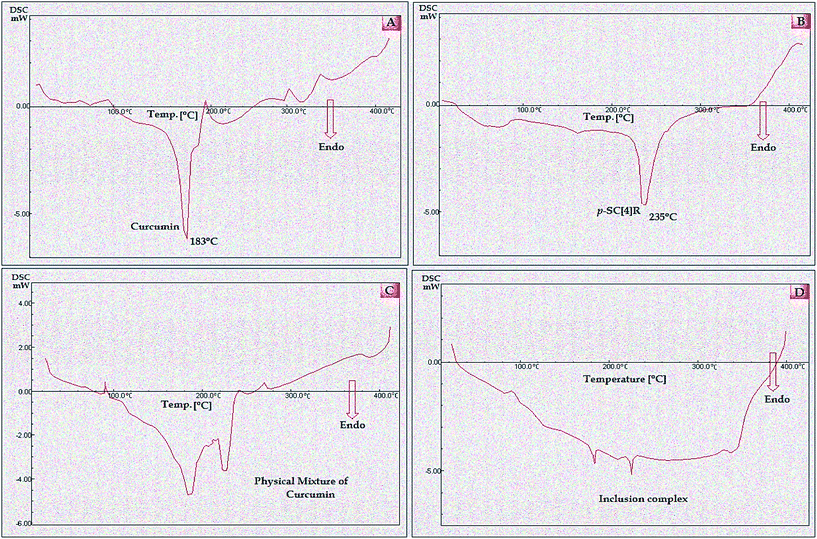 |
| | Fig. 5 DSC thermo grams of (A) pure curcumin, (B) p-SC[4]R, (C) physical mixture and (D) inclusion complex. | |
SEM analysis
SEM analysis is the most ideal qualitative technique for measuring the surface roughness and for obtaining images of the surface texture. SEM not only gives information about amorphisation of curcumin–p-SC[4]R complex but it also shows the uniformity of the amorphous solid at the microscopic level, showing irregularly shaped aragonite crystals of 1 μm width. The morphology of the curcumin and curcumin–p-SC[4]R inclusion complex were evaluated by SEM as shown in Fig. 6. The curcumin exists in extremely rounded spherical and uniform particles, whereas p-SC[4]R appears as a rice-seed like or long rod like morphology, which is observed for most of the precipitated particles. However, the electron micrograph of the physical mixture of the two powders of curcumin–p-SC[4]R exhibited some similarities with the rod like shape of p-SC[4]R and the rounded spheres of curcumin found in combinations of large and small agglomerates. In contrast, the curcumin–p-SC[4]R inclusion complex shows a change in the particle's morphological appearance, to contain neither rounded spherical structures nor rice-seed like patterns but exhibits aggregation into irregularly shaped amorphous particles. This micrograph exposes the development of aragonite mineral aggregates from a central pseudo-hexagonal basal platter. This growth process is the central prime pattern nucleation followed by subsidiary nucleation of a cluster of needle shaped morphology which has an overall comet like appearance. These micrographs clearly elucidate the differences between the substances. Thus, these morphological variations are indicative of the presence of an apparent interaction between curcumin and p-SC[4]R. This complexation suggests a simple methodology not only for the association of multiple drugs but also enhances their pharmaceutics for oral drug delivery.
 |
| | Fig. 6 SEM micrographs of (A) pure curcumin, (B) p-SC[4]R, (C) physical mixture and (D) inclusion complex. | |
ESI-MS analysis
The ESI-MS spectral analysis has shown itself to be a very convenient method in describing the non-covalent interaction between p-SC[4]R and the curcumin guest, owing to its high sensitivity, rapid and low sample consumption. ESI-MS spectra of the solid inclusion complex of curcumin–p-SC[4]R, pure curcumin and p-SC[4]R are shown in Fig. 7. The ESI-MS spectrum of pure curcumin shows three molecular ion peaks with m/z at 368.1, 369.1 and 391.4 corresponding to curcumin mass (M), M + 1 and M + Na, whereas p-SC[4]R shows a peak at 865.3. The positive mode ESI-MS spectra of the inclusion complex shows two peaks at 1232.1 and 1255.4, which are due to p-SC[4]R + curcumin and p-SC[4]R + Na-curcumin, which indeed suggests the presence of binding of curcumin with p-SC[4]R. Moreover, negative mode ESI-MS spectra of the inclusion complex shows two peaks at 1230.8 and 1230.0 due to p-SC[4]R + curcumin − H (M − 1) and p-SC[4]R + curcumin − 2 (M − 2), which also suggests the presence of superficial interactions between curcumin and p-SC[4]R. These results clearly indicate that the binding ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between curcumin and p-SC[4]R during complexation.
1 between curcumin and p-SC[4]R during complexation.
 |
| | Fig. 7 ESI-MS spectra of (A) curcumin, (B) p-SC[4]R and (C) curcumin–p-SC[4]R inclusion complex (ES + mode) as well as ES − mode spectra of inclusion complex (D). | |
Elemental analysis
The analytical calculations of inclusion complex for C%, H%, O% and S% were found to be 51.62%, 4.25%, 33.73% and 10.40%, respectively, whereas practical values of C%, H%, O% and S% were 51.65%, 4.23%, 33.75% and 10.37%, in that order. The values of the curcumin–p-SC[4]R inclusion complex were confirmed mostly based on the data from carbon and oxygen, elucidating that curcumin was completely complexed with the p-SC[4]R in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.
1 stoichiometry.
In vitro evaluations
Dissolution analysis. In vitro dissolution profiles of the curcumin–p-SC[4]R inclusion complex, pure curcumin and the physical mixture of curcumin–p-SC[4]R at the equivalent ratio are shown in Fig. S2 (ESI†). As seen in the Fig. S2,† the curcumin–p-SC[4]R inclusion complex exhibits initially rapid and manifestly superior dissolution release compared to the corresponding physical mixture and pure curcumin with an accumulative dissolution of over 90%. The dissolution process attained a constant value within 30 min and dissolved completely in 40 min. At the end of 3 h, pure curcumin, physical mixture and inclusion complex released were 9.89%, 5.49% and 0.83%, respectively. These results indicate that the rise in solubility, the reduced curcumin crystallite size and an enhanced curcumin wettability lead to the conversion of curcumin to the amorphous state. The pure curcumin showed a sluggish dissolution rate owing to its hydrophobicity that produced the powder to levitate on the surface of the dissolution medium. The physical mixture also resulted in higher dissolution compared to pure curcumin and the dissolution is attributable to the in situ formation of the readily soluble inclusion complex. In short, the results concluded that the curcumin–p-SC[4]R inclusion complex shows considerably faster dissolution than the pure curcumin and this enhanced dissolution leads to an improved oral bioavailability of curcumin.
Effect of temperature on the complexation between curcumin and p-SC[4]R
The phase solubility diagrams of the curcumin–p-SC[4]R inclusion complex, shown in Fig. S3 (ESI†), at different temperatures of 25, 35 & 45 °C were used to calculate the stability constants (Kc) and the thermodynamic values for the formation of the curcumin–p-SC[4]R complexes. All the diagrams show that the solubility of curcumin increases linearly along with the concentration of p-SC[4]R, confirming earlier observations of an AL type diagram suggestive of the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the complex. The intercept, slope and stability constant obtained from the curcumin–p-SC[4]R phase solubility diagrams are given in Table 2, according to the linear regression equation and stability constant (Kc) eqn (1). The increase in the intercept value indicates that the aqueous solubility of curcumin increases with temperature, as well as the total molar concentration of curcumin in aqueous media containing p-SC[4]R increases at higher temperature. However, the stability constant Kc decreases with increasing temperature, representing that the inclusion of curcumin into p-SC[4]R is an exothermic process. Furthermore, some thermodynamic parameters of the inclusion complex could also be obtained from the phase solubility studies (Fig. 10) at different temperatures of 25, 35 and 45 °C. The values of enthalpy (ΔH) and entropy (ΔS) changes were calculated from the stability constant (Kc) using the integrated form of the Van't Hoff eqn (3) at different temperatures. The inclusion complex of curcumin–p-SC[4]R, shown in Fig. 10, exhibits a linear association (R2 = 0.998) between the logarithm of the stability constant (Kc) and the inverse of the absolute temperature (1/T). Thermodynamic parameters ΔH = −24.08 kJ mol−1 and ΔS = −6.29 J mol−1 are calculated from the slope and intercept respectively. Moreover, the free energy change (ΔG) for the inclusion complex formation was calculated by the Gibbs free energy eqn (4). The value of ΔG was found to be −17.79 kJ mol−1. The negative value of enthalpy change (ΔH) shows that the interaction processes between curcumin and p-SC[4]R were exothermic. These interaction processes may consist of the displacement of water molecules from the cavity of the p-SC[4]R by the more hydrophobic curcumin with the formation of hydrogen bonds or other low energy interactions and an increase in van der Waals interactions between molecules. The entropy change (ΔS) is negative, indicating that complexation resulted in the system's environment becoming more stable and orderly, owing to the decrease in the translational and rotational degrees of the curcumin compared to p-SC[4]R in the complexed state. Overall, the ΔG negative value indicates that the formation of curcumin–p-SC[4]R inclusion complex is a spontaneous processes.
1 stoichiometry of the complex. The intercept, slope and stability constant obtained from the curcumin–p-SC[4]R phase solubility diagrams are given in Table 2, according to the linear regression equation and stability constant (Kc) eqn (1). The increase in the intercept value indicates that the aqueous solubility of curcumin increases with temperature, as well as the total molar concentration of curcumin in aqueous media containing p-SC[4]R increases at higher temperature. However, the stability constant Kc decreases with increasing temperature, representing that the inclusion of curcumin into p-SC[4]R is an exothermic process. Furthermore, some thermodynamic parameters of the inclusion complex could also be obtained from the phase solubility studies (Fig. 10) at different temperatures of 25, 35 and 45 °C. The values of enthalpy (ΔH) and entropy (ΔS) changes were calculated from the stability constant (Kc) using the integrated form of the Van't Hoff eqn (3) at different temperatures. The inclusion complex of curcumin–p-SC[4]R, shown in Fig. 10, exhibits a linear association (R2 = 0.998) between the logarithm of the stability constant (Kc) and the inverse of the absolute temperature (1/T). Thermodynamic parameters ΔH = −24.08 kJ mol−1 and ΔS = −6.29 J mol−1 are calculated from the slope and intercept respectively. Moreover, the free energy change (ΔG) for the inclusion complex formation was calculated by the Gibbs free energy eqn (4). The value of ΔG was found to be −17.79 kJ mol−1. The negative value of enthalpy change (ΔH) shows that the interaction processes between curcumin and p-SC[4]R were exothermic. These interaction processes may consist of the displacement of water molecules from the cavity of the p-SC[4]R by the more hydrophobic curcumin with the formation of hydrogen bonds or other low energy interactions and an increase in van der Waals interactions between molecules. The entropy change (ΔS) is negative, indicating that complexation resulted in the system's environment becoming more stable and orderly, owing to the decrease in the translational and rotational degrees of the curcumin compared to p-SC[4]R in the complexed state. Overall, the ΔG negative value indicates that the formation of curcumin–p-SC[4]R inclusion complex is a spontaneous processes.
Table 2 The intercept, slope, stability constant and Gibbs free energy obtained from curcumin–p-SC[4]R phase solubility diagrams at different temperatures
| Temperature (°C) |
Intercept (×10−6 M) |
Slope |
R2 |
Stability constant (Kc, M−1) |
ΔG (kJ mol−1) |
| 25 |
0.15 |
0.985 |
0.99 |
985.00 |
−17.09 |
| 35 |
0.91 |
0.993 |
0.99 |
772.33 |
−17.04 |
| 45 |
1.71 |
0.999 |
0.99 |
587.65 |
−16.87 |
 |
| | Fig. 10 Van't Hoff plot for curcumin–p-SC[4]R inclusion complex. | |
Stability of free curcumin and curcumin–p-SC[4]R complex
Storage stability. In the present study, the potential of p-SC[4]R to protect curcumin from light, heat and UV loss was investigated. The degradation of curcumin and its inclusion complex with p-SC[4]R complex when exposed to light, heat and UV radiation over 40 days period is shown in Fig. S4 (ESI†). The stability of curcumin to light exposure has been reported earlier.8 For curcumin exposed to light, heat and UV, all groups of curcumin free and complexed forms were gradually degraded, and the degradation occurred at a slower rate for curcumin in the complex form. Thus, light, heat and UV appear to interfere significantly with the integrity of colorant curcumin. After 40 days, the amount of curcumin remaining was 57% and 78% for the free and complexed forms, respectively. Measurement of curcumin colour intensity after being stored under dark conditions showed that the difference between the complexed and uncomplexed curcumin was small, suggesting a protective action by p-SC[4]R on curcumin against light, heat and UV irradiation. As a result, the improvement in the stability of curcumin by complexing with p-SC[4]R is exceedingly important in the food and pharmaceutical fields.
pH stability. Fig. S5 (ESI†) shows the stability results of the pure and complexed curcumin within the pH range from 2–12. The curcumin–p-SC[4]R inclusion complex displays greater stability compared to the pure colorant for the pH range of 1–7. However, at pH values 8–12, degradation occurs for pure curcumin as well as the curcumin complex and both the solutions visually change from yellow to red. Curcumin is exposed to hydrolytic degradative reactions in basic media and is therefore unstable in an alkali medium; therefore it is more suitable to distinguish curcumin in the media whose pH value is maintained below 7.28 However, in an acidic medium, the colorant absorbance is steadily increases and slowly decreases in alkali medium compared to the pure curcumin. This indicates that the chemical stability of curcumin is also greatly improved when the curcumin–p-SC[4]R inclusion complex is formed.
Anti-oxidant activity of curcumin in free and complex form
The anti-oxidant activities of curcumin and the inclusion complex of curcumin–p-SC[4]R, measured by their DPPH radical scavenging activity, was compared to that of standard ascorbic acid (Fig. 11). DPPH is a stable free radical and proton donating substance, generating a deep violet colour in organic solvent. Its progressive discoloration in the presence of curcumin indicates that it is acting as an anti-oxidant. The reduction capability was determined by monitoring the decrease in absorption by the DPPH radical at 516 nm. The effect of curcumin, p-SC[4]R and the curcumin–p-SC[4]R inclusion complex on DPPH staining was measured quantitatively from 0.5 to 10 μg mL−1. The % of DPPH radical scavenging is increased by increasing the concentration of pure curcumin, p-SC[4]R and its complex with p-SC[4]R as well as ascorbic acid (Fig. 11). The calculated inhibitory concentration (IC50) value of curcumin and the inclusion complex of curcumin–p-SC[4]R are 3.93 ± 0.03 μg mL−1 and 3.56 ± 0.07 μg mL−1, respectively, as compared to the IC50 value of ascorbic acid for DPPH radical, which is 4.31 ± 0.08 μg mL−1. However, the calculated inhibitory concentration of p-SC[4]R is 3.69 ± 0.05, due to calix[4]resorcinarene, which can transfer electrons or hydrogen atoms to the non-radical form of DPPH and is a highly efficient antioxidant agent.29 This means curcumin in complex form with a concentration of only 3.56 ± 0.07 μg mL−1 is required to scavenge 50% of DPPH radicals whereas the concentration of curcumin free and standard ascorbic acid needed to scavenge the same amount of the radicals were 3.93 μg mL−1 and 4.31 μg mL−1, respectively. These results clearly indicate that curcumin in the presence of p-SC[4]R is a powerful free radical inhibitor. These results also indicate that complex formation could be useful to maintain and even enhance the anti-oxidant activity of curcumin.
 |
| | Fig. 11 Anti-oxidant activity of curcumin, p-SC[4]R, curcumin–p-SC[4]R inclusion complex and ascorbic acid as determined by DPPH radical scavenging method. | |
In vivo acute toxicity study
From the in vivo acute oral toxicity studies, LD50 of the solid curcumin–p-SC[4]R inclusion complex was found to be greater than 2500 mg kg−1 of body weight in Swiss albino mice. In mice, a single dose of free para-sulfonatocalixarene at doses equivalent to 2–5 g kg−1 in humans shows no acute toxicity.30 At the end of the 14 days period, all the surviving animals were weighed, and no substantial changes were observed as compared to the initial weight on the 1st day. The animals appeared active and healthy during the study. During the acute oral toxicity study and body weight measurements, the animals did not show any toxic effects. Apart from the toxicity observations, there were no signs of gross toxicity in the mice. An autopsy at the end of the study did not reveal any gross pathological abnormalities or adverse pharmacological effects in any mice. Therefore, the abovementioned study indicates that curcumin–p-SC[4]R is non-toxic to an oral dose LD50 of about 2750 mg kg−1 (Table 3) of body weight in Swiss albino mice. The solid lipid curcumin particle earlier reported was found to have an oral LD50 in rats as well as in mice which was found to be 2000 mg kg−1 of body weight for a dose of 720 mg kg−1.28
Table 3 In vivo oral acute toxicity study of Swiss albino mice injected with the inclusion complex
| Animal |
Time (h) |
Dose (mg kg−1) |
Survivala |
| √: animal survived, ×: animal died. |
| 1 |
12 |
500 |
√ |
| 24 |
√ |
| 36 |
√ |
| 48 |
√ |
| 2 |
12 |
1000 |
√ |
| 24 |
√ |
| 36 |
√ |
| 48 |
√ |
| 3 |
12 |
1500 |
√ |
| 24 |
√ |
| 36 |
√ |
| 48 |
√ |
| 4 |
12 |
2000 |
√ |
| 24 |
√ |
| 36 |
√ |
| 48 |
√ |
| 5 |
12 |
2500 |
√ |
| 24 |
√ |
| 36 |
√ |
| 48 |
√ |
| 6 |
12 |
2750 |
√ |
| 24 |
√ |
| 36 |
√ |
| 48 |
√ |
| 7 |
12 |
3000 |
× |
| 24 |
– |
| 36 |
– |
| 48 |
– |
Conclusion
In summary, we have successfully prepared the inclusion complex of curcumin–p-SC[4]R at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation stoichiometry with 985 mM−1 stability constant and also confirmed by Job's plot method (R = 0.5). The evidence was obtained by various spectroscopic methods, including UV, FT-IR, HPLC, DSC, SEM, PXRD, 1H NMR and ESI-mass analysis. From the temperature dependent studies, negative values for the thermodynamic parameters ΔS & ΔH indicate that the formation of the inclusion complex of curcumin–p-SC[4]R was an exothermic process, which occurred spontaneously. The formation of such an inclusion complex offered moderate degrees of protection to curcumin from light, thermal & UV radiation degradation during storage, and the pH stability of the inclusion complex is better in acidic media in comparison of curcumin alone. The DPPH scavenging capacity of curcumin on complexation with p-SC[4]R was enhanced compared to pure curcumin. Moreover, in vivo oral acute toxicity showed that there are remarkable changes in the toxicity of curcumin alone indicating that the pure curcumin becomes less toxic, as the LD50 value increases (2750 mg kg−1) as compared to the previously reported LD50 value (2000 mg kg−1) of the curcumin solid lipid particle after complexation. All these results concluded that p-SC[4]R complexation of curcumin takes place in the hydrophobic cavity of p-SC[4]R. Further research could examine the chemical, biological and processing properties and their application in pharmaceutical and functional foods.
1 complexation stoichiometry with 985 mM−1 stability constant and also confirmed by Job's plot method (R = 0.5). The evidence was obtained by various spectroscopic methods, including UV, FT-IR, HPLC, DSC, SEM, PXRD, 1H NMR and ESI-mass analysis. From the temperature dependent studies, negative values for the thermodynamic parameters ΔS & ΔH indicate that the formation of the inclusion complex of curcumin–p-SC[4]R was an exothermic process, which occurred spontaneously. The formation of such an inclusion complex offered moderate degrees of protection to curcumin from light, thermal & UV radiation degradation during storage, and the pH stability of the inclusion complex is better in acidic media in comparison of curcumin alone. The DPPH scavenging capacity of curcumin on complexation with p-SC[4]R was enhanced compared to pure curcumin. Moreover, in vivo oral acute toxicity showed that there are remarkable changes in the toxicity of curcumin alone indicating that the pure curcumin becomes less toxic, as the LD50 value increases (2750 mg kg−1) as compared to the previously reported LD50 value (2000 mg kg−1) of the curcumin solid lipid particle after complexation. All these results concluded that p-SC[4]R complexation of curcumin takes place in the hydrophobic cavity of p-SC[4]R. Further research could examine the chemical, biological and processing properties and their application in pharmaceutical and functional foods.
Acknowledgements
Nikunj N. Valand gratefully acknowledges DST, New Delhi for the INSPIRE Junior Research Fellowship. Prof. N K Jain, Flora Shah, Namrata Bhagia (Dept. of Life Science) and Kalpesh Solanki (Dept. of Forensic Science) are acknowledged for their help in HPLC studies.
References
- JECFA, Safety Evaluation of Certain Food Additives & Contaminants, WHO, 2004, Prepared by the 61st Meeting of JECFA.WHO Food Additive Series: 52, pp. 55–60 Search PubMed.
- H. Hatcher, R. Planalp, J. Cho, F. M. Torti and S. V. Torti, Cell. Mol. Life Sci., 2008, 65, 1631–1652 CrossRef CAS PubMed.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharm., 2007, 4, 807–818 CrossRef CAS PubMed.
- K. Maiti, K. Mukherjee, A. Gantait, B. P. Saha and P. K. Mukherjee, Int. J. Pharm., 2007, 330, 155–163 CrossRef CAS PubMed.
- Bhawana, R. K. Basniwal, H. S. Buttar, V. K. Jain and N. Jain, J. Agric. Food Chem., 2011, 59, 2056–2061 CrossRef CAS PubMed.
- H. Yu and Q. Huang, J. Agric. Food Chem., 2012, 60, 5373–5379 CrossRef CAS PubMed.
- A. Safavy, K. P. Raisch, S. Mantena, L. L. Sanford, S. W. Sham, N. R. Krishna and J. A. Bonner, J. Med. Chem., 2007, 50, 6284–6288 CrossRef CAS PubMed.
- V. A. Marcolino, G. M. Zanin, L. R. Durrant, M. D. T. Benassi and G. Matioli, J. Agric. Food Chem., 2011, 59, 3348–3357 CrossRef CAS PubMed.
- A. L. Koner, I. Ghosh, N. I. Saleh and W. M. Nau, Can. J. Chem., 2011, 89, 139–147 CrossRef CAS.
- A. Mukerjee and J. K. Vishvanatha, Anticancer Res., 2009, 29, 3867–3876 CAS.
- T. Harada, D. T. Pham, M. H. M. Leung, H. T. Ngo, S. F. Lincoln, C. J. Easton and T. W. Kee, J. Phys. Chem. B, 2011, 115, 1268–1274 CrossRef CAS PubMed.
- R. Lalor, H. Baillie-Johnson, C. Redshaw, S. E. Matthews and A. Mueller, J. Am. Chem. Soc., 2008, 130, 2892–2893 CrossRef CAS PubMed.
- F. Perret, A. N. Lazar and A. W. Coleman, Chem. Commun., 2006, 2425–2438 RSC.
- P. M. Mareeswaran, E. Babu, V. Sathish, B. Kim, S. I. Woo and S. Rajagopal, New J. Chem., 2014, 38, 1336–1345 RSC.
-
(a) V. Böhmer, Calixarenes, Macrocycles with (Almost) Unlimited Possibilities, Angew. Chem., Int. Ed., 1995, 34, 713–745 CrossRef;
(b) C. D. Gutsche, in Calixarenes Revisited, ed. J. F. Stoddart, The Royal Society of Chemistry, Cambridge, UK, 1998, Monographs in Supramolecular Chemistry Search PubMed;
(c) A. Ikeda and S. Shinkai, Chem. Rev., 1997, 97, 1713–1734 CrossRef CAS PubMed.
- S. Shinkai, K. Araki, T. Matsuda, N. Nishiyama, H. Ikeda, I. Takasu and M. Iwamoto, J. Am. Chem. Soc., 1990, 112, 9053–9058 CrossRef CAS.
- E. Da Silva, A. N. Lazar and A. W. Coleman, J. Drug Delivery Sci. Technol., 2004, 14, 3–20 CrossRef CAS.
-
(a) P. Timmerman, W. Verboom and D. N. Reinhoudt, Tetrahedron, 1996, 52, 2663–2704 CrossRef CAS;
(b) A. G. S. Hogberg, J. Org. Chem., 1980, 45, 4498–4500 CrossRef.
- K. Helttunen, K. Salorinne, T. Barboza, H. C. Barbosa, A. Suhonen and M. Nissinen, New J. Chem., 2012, 36, 789–795 RSC.
- L. Mandolini and R. Ungaro, Calixarenes in Action, Imperial College Press, Singapore, 2000, p. 271 Search PubMed.
- Z. Asfari, V. Böhmer, J. Harrowfield and J. Vicens, Calixarenes 2001, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2001, p. 683 Search PubMed.
- F. Perret and A. W. Coleman, Chem. Commun., 2011, 47, 7303–7319 RSC.
- M. B. Patel, N. N. Valand, N. R. Modi, K. V. Joshi, U. Harikrishnan, S. Prasanth Kumar, Y. T. Jasrai and S. K. Menon, RSC Adv., 2013, 3, 15971–15981 RSC.
- T. Higuchi and K. A. Connors, Adv. Anal. Chem. Instrum., 1965, 4, 117–212 CAS.
- V. Venuti, C. Cannavà, M. C. Cristiano, M. Fresta, D. Majolino, D. Paolino, R. Stancanelli, S. Tommasini and C. A. Ventura, Colloids Surf., B, 2014, 115, 22–28 CrossRef CAS PubMed.
- T. A. Nguyen, B. Liu, J. Zhao, D. S. Thomas and J. M. Hook, Food Chem., 2013, 136, 186–192 CrossRef CAS PubMed.
- C. S. Mangolim, C. Moriwaki, A. C. Nogueira, F. Sato, M. L. Baesso, A. M. Neto and G. Matioli, Food Chem., 2014, 153, 361–370 CrossRef CAS PubMed.
- P. Dadhaniya, C. Patel, J. Muchhara, N. Bhadja, N. Mathuria, K. Vachhani and M. G. Soni, Food Chem. Toxicol., 2011, 49, 1834–1842 CrossRef CAS PubMed.
-
(a) A. I. Vovk, A. M. Shivanyuk, R. V. Bugas, O. V. Muzychka and A. K. Melnyk, Bioorg. Med. Chem. Lett., 2009, 19, 1314–1317 CrossRef CAS PubMed;
(b) A. Hasbullah, H. M. Abosadiya, Jumina, M. I. M. Tahir and B. M. Yamin, International journal on Advanced Science Engineering Information Technology, 2013, 3, 2 Search PubMed.
- A. W. Coleman, S. Jebors, S. Cecillon, P. Perret, D. Garin, D. M. Battle and M. Moulin, New J. Chem., 2008, 32, 780–782 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S6. See DOI: 10.1039/c4ra12047g |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex formation of curcumin–p-SC[4]R has been investigated with the aim to enhance the solubility, bioavailability, stability and anti-oxidant activity as well as decrease in vivo acute oral toxicity of curcumin by inclusion complexation. Thermodynamic parameters ΔS and ΔH are negative, indicating that the inclusion complexation was an exothermic process, which occurred spontaneously. The inclusion complex was characterised by different analytical methods, including FT-IR, PXRD, 1H-NMR, SEM, DSC, ESI-mass, UV-Vis spectroscopy and elemental analysis.
1 stoichiometric complex formation of curcumin–p-SC[4]R has been investigated with the aim to enhance the solubility, bioavailability, stability and anti-oxidant activity as well as decrease in vivo acute oral toxicity of curcumin by inclusion complexation. Thermodynamic parameters ΔS and ΔH are negative, indicating that the inclusion complexation was an exothermic process, which occurred spontaneously. The inclusion complex was characterised by different analytical methods, including FT-IR, PXRD, 1H-NMR, SEM, DSC, ESI-mass, UV-Vis spectroscopy and elemental analysis.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50%, v/v) (containing 3% glacial acetic acid) filtered with 0.45 μm membrane filter paper at 1.0 mL min−1 flow rate. DSC analyses of all samples were performed in a Shimadzu model DSC-60 (Japan) calibrated with indium. SEM microscopy of curcumin, p-SC[4]R, a physical mixture of curcumin–p-SC[4]R and the inclusion complex were performed with a Leo 440i, Leo Electron Microscopy Ltd, Cambridge CB1 3QH, England, to visualize the surface morphology.
50%, v/v) (containing 3% glacial acetic acid) filtered with 0.45 μm membrane filter paper at 1.0 mL min−1 flow rate. DSC analyses of all samples were performed in a Shimadzu model DSC-60 (Japan) calibrated with indium. SEM microscopy of curcumin, p-SC[4]R, a physical mixture of curcumin–p-SC[4]R and the inclusion complex were performed with a Leo 440i, Leo Electron Microscopy Ltd, Cambridge CB1 3QH, England, to visualize the surface morphology.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of curcumin (0.368 g, 1 mM) and p-SC[4]R (0.865 g, 1 mM) were dissolved in 100 mL of ultrapure water, stirred for 48 h at 37 °C by rotary shaker in the dark and filtered through 0.45 μm membrane filter to eliminate undissolved materials. The filtrate was dried at 60 °C under 2–5 torr vacuum to collect the inclusion complex of curcumin and p-SC[4]R (Scheme 1).
1 molar ratio of curcumin (0.368 g, 1 mM) and p-SC[4]R (0.865 g, 1 mM) were dissolved in 100 mL of ultrapure water, stirred for 48 h at 37 °C by rotary shaker in the dark and filtered through 0.45 μm membrane filter to eliminate undissolved materials. The filtrate was dried at 60 °C under 2–5 torr vacuum to collect the inclusion complex of curcumin and p-SC[4]R (Scheme 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio was prepared by simple grinding using an agate mortar and pestle to obtain a uniform physical mixture.
1 molar ratio was prepared by simple grinding using an agate mortar and pestle to obtain a uniform physical mixture.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex, the apparent stability constants, Kc, were calculated from the slope and intercept, So, of the initial straight line portion of the phase solubility diagrams using eqn (1) given as follows:
1 inclusion complex, the apparent stability constants, Kc, were calculated from the slope and intercept, So, of the initial straight line portion of the phase solubility diagrams using eqn (1) given as follows:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; v/v) solutions of curcumin and p-SC[4]R were mixed to a fixed volume by varying the molar ratio from 0.1 to 1, keeping the total concentration of solutes constant. After 1 h of stirring, the absorbance of each solution was measured by UV-Vis spectroscopy at 428 nm. The difference between the absorbance of curcumin with and without p-SC[4]R was plotted against R, where R is obtained by eqn (2) given as follows:
50; v/v) solutions of curcumin and p-SC[4]R were mixed to a fixed volume by varying the molar ratio from 0.1 to 1, keeping the total concentration of solutes constant. After 1 h of stirring, the absorbance of each solution was measured by UV-Vis spectroscopy at 428 nm. The difference between the absorbance of curcumin with and without p-SC[4]R was plotted against R, where R is obtained by eqn (2) given as follows:
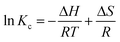
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc
Kc
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% (v/v). The solutions were prepared just before taking the measurements. The 5 mL solutions, were adjusted to pH values in the range of 2–12 using buffer solutions27 and the wavelength of absorbance was determined at 428 nm by a Jasco V-570 spectrophotometer.
50% (v/v). The solutions were prepared just before taking the measurements. The 5 mL solutions, were adjusted to pH values in the range of 2–12 using buffer solutions27 and the wavelength of absorbance was determined at 428 nm by a Jasco V-570 spectrophotometer.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50%; v/v) and appropriately diluted. A 0.3 mM (0.013 g mL−1) of DPPH solution was prepared in methanol–water (50%
50%; v/v) and appropriately diluted. A 0.3 mM (0.013 g mL−1) of DPPH solution was prepared in methanol–water (50%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50%; v/v). Then, 2 mL of DPPH solution were added to 2 mL of sample solutions at different concentration and the mixtures were incubated at room temperature for 30 min. The free radical scavenging activity of the inclusion complex as well as pure curcumin and p-SC[4]R were measured by monitoring the decay of absorbance of the DPPH solution at 518 nm in the presence of the inclusion complex as well as p-SC[4]R and curcumin alone using a Jasco V-570 UV-Vis spectrophotometer. The anti-oxidant activity of the solutions as well as that of ascorbic acid was assayed by the same procedure for comparison. The experiment was performed in triplicate. DPPH scavenging activity was calculated as per the eqn (5) given as follows:
50%; v/v). Then, 2 mL of DPPH solution were added to 2 mL of sample solutions at different concentration and the mixtures were incubated at room temperature for 30 min. The free radical scavenging activity of the inclusion complex as well as pure curcumin and p-SC[4]R were measured by monitoring the decay of absorbance of the DPPH solution at 518 nm in the presence of the inclusion complex as well as p-SC[4]R and curcumin alone using a Jasco V-570 UV-Vis spectrophotometer. The anti-oxidant activity of the solutions as well as that of ascorbic acid was assayed by the same procedure for comparison. The experiment was performed in triplicate. DPPH scavenging activity was calculated as per the eqn (5) given as follows:
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1605 (–C
O), 1605 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration), 1459 (–C–O), 1191 (–SO3H), 1026 (–C–O–C– stretching). 1H NMR (400 MHz, DMSO-d6): δ = 3.813 (S, 6H, J = 11.5 Hz, –OCH3), 4.523 (S, 2H, J = 12.40, –CH methylidine), 4.104 (m, 4H, J = 6.4 Hz, –CH), 6.627 (d, 2H, –CH), 6.751 (d, 2H, –CH), 6.784 (S, 2H, –CH), 6.883 (S, 2H, –CH), 7.090 (S, 4H, Ar-H), 7.431 (d, 2H, –CH), 7.562 (S, 2H, –OH), 9.891 (S, 4H, –SO3H), 8.451 (S, 8H, –OH, p-SC[4]R), 1.247 (d, 12H, J = 6.8 Hz, –CH3). ESI-MS m/z 1232.1 (Curcumin + p-SC[4]R).
C stretching vibration), 1459 (–C–O), 1191 (–SO3H), 1026 (–C–O–C– stretching). 1H NMR (400 MHz, DMSO-d6): δ = 3.813 (S, 6H, J = 11.5 Hz, –OCH3), 4.523 (S, 2H, J = 12.40, –CH methylidine), 4.104 (m, 4H, J = 6.4 Hz, –CH), 6.627 (d, 2H, –CH), 6.751 (d, 2H, –CH), 6.784 (S, 2H, –CH), 6.883 (S, 2H, –CH), 7.090 (S, 4H, Ar-H), 7.431 (d, 2H, –CH), 7.562 (S, 2H, –OH), 9.891 (S, 4H, –SO3H), 8.451 (S, 8H, –OH, p-SC[4]R), 1.247 (d, 12H, J = 6.8 Hz, –CH3). ESI-MS m/z 1232.1 (Curcumin + p-SC[4]R).

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups, and 1603 cm−1 is indicative of the symmetric aromatic stretching vibration. The sharp peak at 1455 cm−1 is attributed to the phenolic C–O, whereas the enolic C–O peak was obtained at 1275 cm−1. Absorption at 1025 cm−1 is indicative of the C–O–C asymmetric stretching vibration. The peak at 958 cm−1 is due to the benzoate trans C–H vibration and at 712 cm−1 is the C–H vibration of the aromatic ring. The FT-IR spectrum of p-SC[4]R consists of broad absorption bands of –OH stretching at 3456 cm−1, whereas three characteristic absorption peaks at 1187, 1115 and 1057 cm−1 are indicative of the –SO3 group. The FT-IR spectra of the physical mixture is found to have merged with lower intense broad OH peak due to the interactions with supramolecule during complex formation, and moreover, all the sharp peaks of curcumin as well as p-SC[4]R are observed along with a few other peaks and reveal reduced sharpness indicating that the spectrum of the physical mixture was essentially a combination of the spectra of the two molecules of curcumin with p-SC[4]R. However, the curcumin–p-SC[4]R inclusion complex spectrum shows no features similar to that of pure curcumin. Several insignificant absorption peaks of curcumin from 500–1200 cm−1 almost disappeared and the sharp –OH stretching band of curcumin at 3510 cm−1 broadened, which indicates the inclusion of curcumin into the p-SC[4]R cavity.
C groups, and 1603 cm−1 is indicative of the symmetric aromatic stretching vibration. The sharp peak at 1455 cm−1 is attributed to the phenolic C–O, whereas the enolic C–O peak was obtained at 1275 cm−1. Absorption at 1025 cm−1 is indicative of the C–O–C asymmetric stretching vibration. The peak at 958 cm−1 is due to the benzoate trans C–H vibration and at 712 cm−1 is the C–H vibration of the aromatic ring. The FT-IR spectrum of p-SC[4]R consists of broad absorption bands of –OH stretching at 3456 cm−1, whereas three characteristic absorption peaks at 1187, 1115 and 1057 cm−1 are indicative of the –SO3 group. The FT-IR spectra of the physical mixture is found to have merged with lower intense broad OH peak due to the interactions with supramolecule during complex formation, and moreover, all the sharp peaks of curcumin as well as p-SC[4]R are observed along with a few other peaks and reveal reduced sharpness indicating that the spectrum of the physical mixture was essentially a combination of the spectra of the two molecules of curcumin with p-SC[4]R. However, the curcumin–p-SC[4]R inclusion complex spectrum shows no features similar to that of pure curcumin. Several insignificant absorption peaks of curcumin from 500–1200 cm−1 almost disappeared and the sharp –OH stretching band of curcumin at 3510 cm−1 broadened, which indicates the inclusion of curcumin into the p-SC[4]R cavity.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between curcumin and p-SC[4]R during complexation.
1 between curcumin and p-SC[4]R during complexation.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry.
1 stoichiometry.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 curcumin–p-SC[4]R stoichiometry. The apparent stability constant (Kc) of the inclusion complex was calculated from the slope of the linear plot of the curves according to eqn (1). The apparent stability constant value of Kc (1
1 curcumin–p-SC[4]R stoichiometry. The apparent stability constant (Kc) of the inclusion complex was calculated from the slope of the linear plot of the curves according to eqn (1). The apparent stability constant value of Kc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was found to be 985 mM−1, indicating that the complex formed between curcumin–p-SC[4]R was quite stable. In fact, the apparent stability constant values are always within the range of 100 to 1000 mM−1.23 These results indicate the formation of a stable 1
1) was found to be 985 mM−1, indicating that the complex formed between curcumin–p-SC[4]R was quite stable. In fact, the apparent stability constant values are always within the range of 100 to 1000 mM−1.23 These results indicate the formation of a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex between curcumin and the polar cavity of p-SC[4]R.
1 inclusion complex between curcumin and the polar cavity of p-SC[4]R.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. These results are in agreement with the phase solubility studies.
1 stoichiometry. These results are in agreement with the phase solubility studies.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the complex. The intercept, slope and stability constant obtained from the curcumin–p-SC[4]R phase solubility diagrams are given in Table 2, according to the linear regression equation and stability constant (Kc) eqn (1). The increase in the intercept value indicates that the aqueous solubility of curcumin increases with temperature, as well as the total molar concentration of curcumin in aqueous media containing p-SC[4]R increases at higher temperature. However, the stability constant Kc decreases with increasing temperature, representing that the inclusion of curcumin into p-SC[4]R is an exothermic process. Furthermore, some thermodynamic parameters of the inclusion complex could also be obtained from the phase solubility studies (Fig. 10) at different temperatures of 25, 35 and 45 °C. The values of enthalpy (ΔH) and entropy (ΔS) changes were calculated from the stability constant (Kc) using the integrated form of the Van't Hoff eqn (3) at different temperatures. The inclusion complex of curcumin–p-SC[4]R, shown in Fig. 10, exhibits a linear association (R2 = 0.998) between the logarithm of the stability constant (Kc) and the inverse of the absolute temperature (1/T). Thermodynamic parameters ΔH = −24.08 kJ mol−1 and ΔS = −6.29 J mol−1 are calculated from the slope and intercept respectively. Moreover, the free energy change (ΔG) for the inclusion complex formation was calculated by the Gibbs free energy eqn (4). The value of ΔG was found to be −17.79 kJ mol−1. The negative value of enthalpy change (ΔH) shows that the interaction processes between curcumin and p-SC[4]R were exothermic. These interaction processes may consist of the displacement of water molecules from the cavity of the p-SC[4]R by the more hydrophobic curcumin with the formation of hydrogen bonds or other low energy interactions and an increase in van der Waals interactions between molecules. The entropy change (ΔS) is negative, indicating that complexation resulted in the system's environment becoming more stable and orderly, owing to the decrease in the translational and rotational degrees of the curcumin compared to p-SC[4]R in the complexed state. Overall, the ΔG negative value indicates that the formation of curcumin–p-SC[4]R inclusion complex is a spontaneous processes.
1 stoichiometry of the complex. The intercept, slope and stability constant obtained from the curcumin–p-SC[4]R phase solubility diagrams are given in Table 2, according to the linear regression equation and stability constant (Kc) eqn (1). The increase in the intercept value indicates that the aqueous solubility of curcumin increases with temperature, as well as the total molar concentration of curcumin in aqueous media containing p-SC[4]R increases at higher temperature. However, the stability constant Kc decreases with increasing temperature, representing that the inclusion of curcumin into p-SC[4]R is an exothermic process. Furthermore, some thermodynamic parameters of the inclusion complex could also be obtained from the phase solubility studies (Fig. 10) at different temperatures of 25, 35 and 45 °C. The values of enthalpy (ΔH) and entropy (ΔS) changes were calculated from the stability constant (Kc) using the integrated form of the Van't Hoff eqn (3) at different temperatures. The inclusion complex of curcumin–p-SC[4]R, shown in Fig. 10, exhibits a linear association (R2 = 0.998) between the logarithm of the stability constant (Kc) and the inverse of the absolute temperature (1/T). Thermodynamic parameters ΔH = −24.08 kJ mol−1 and ΔS = −6.29 J mol−1 are calculated from the slope and intercept respectively. Moreover, the free energy change (ΔG) for the inclusion complex formation was calculated by the Gibbs free energy eqn (4). The value of ΔG was found to be −17.79 kJ mol−1. The negative value of enthalpy change (ΔH) shows that the interaction processes between curcumin and p-SC[4]R were exothermic. These interaction processes may consist of the displacement of water molecules from the cavity of the p-SC[4]R by the more hydrophobic curcumin with the formation of hydrogen bonds or other low energy interactions and an increase in van der Waals interactions between molecules. The entropy change (ΔS) is negative, indicating that complexation resulted in the system's environment becoming more stable and orderly, owing to the decrease in the translational and rotational degrees of the curcumin compared to p-SC[4]R in the complexed state. Overall, the ΔG negative value indicates that the formation of curcumin–p-SC[4]R inclusion complex is a spontaneous processes.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation stoichiometry with 985 mM−1 stability constant and also confirmed by Job's plot method (R = 0.5). The evidence was obtained by various spectroscopic methods, including UV, FT-IR, HPLC, DSC, SEM, PXRD, 1H NMR and ESI-mass analysis. From the temperature dependent studies, negative values for the thermodynamic parameters ΔS & ΔH indicate that the formation of the inclusion complex of curcumin–p-SC[4]R was an exothermic process, which occurred spontaneously. The formation of such an inclusion complex offered moderate degrees of protection to curcumin from light, thermal & UV radiation degradation during storage, and the pH stability of the inclusion complex is better in acidic media in comparison of curcumin alone. The DPPH scavenging capacity of curcumin on complexation with p-SC[4]R was enhanced compared to pure curcumin. Moreover, in vivo oral acute toxicity showed that there are remarkable changes in the toxicity of curcumin alone indicating that the pure curcumin becomes less toxic, as the LD50 value increases (2750 mg kg−1) as compared to the previously reported LD50 value (2000 mg kg−1) of the curcumin solid lipid particle after complexation. All these results concluded that p-SC[4]R complexation of curcumin takes place in the hydrophobic cavity of p-SC[4]R. Further research could examine the chemical, biological and processing properties and their application in pharmaceutical and functional foods.
1 complexation stoichiometry with 985 mM−1 stability constant and also confirmed by Job's plot method (R = 0.5). The evidence was obtained by various spectroscopic methods, including UV, FT-IR, HPLC, DSC, SEM, PXRD, 1H NMR and ESI-mass analysis. From the temperature dependent studies, negative values for the thermodynamic parameters ΔS & ΔH indicate that the formation of the inclusion complex of curcumin–p-SC[4]R was an exothermic process, which occurred spontaneously. The formation of such an inclusion complex offered moderate degrees of protection to curcumin from light, thermal & UV radiation degradation during storage, and the pH stability of the inclusion complex is better in acidic media in comparison of curcumin alone. The DPPH scavenging capacity of curcumin on complexation with p-SC[4]R was enhanced compared to pure curcumin. Moreover, in vivo oral acute toxicity showed that there are remarkable changes in the toxicity of curcumin alone indicating that the pure curcumin becomes less toxic, as the LD50 value increases (2750 mg kg−1) as compared to the previously reported LD50 value (2000 mg kg−1) of the curcumin solid lipid particle after complexation. All these results concluded that p-SC[4]R complexation of curcumin takes place in the hydrophobic cavity of p-SC[4]R. Further research could examine the chemical, biological and processing properties and their application in pharmaceutical and functional foods.