Deep-blue emitting pyrene–benzimidazole conjugates for solution processed organic light-emitting diodes†
Abstract
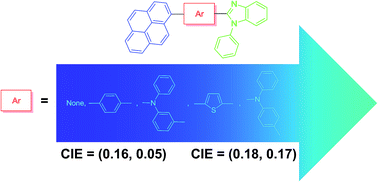
New pyrene–benzimidazole conjugates containing different π-linkers such as phenyl, thiophene and triarylamine were synthesized and characterized by photophysical, electrochemical, thermal and electroluminescence studies. Triarylamine-containing dyes displayed red-shifted absorption spectra and positive solvatochromism in emission spectra due to the pronounced intramolecular charge transfer (ICT) from the triarylamine donor to pyrene acceptor in the excited state. All derivatives were used as emitting dopants in multilayered organic light-emitting diodes exhibiting deep blue electroluminescence. The solution processed device fabricated by utilizing 1-phenyl-2-(pyren-1-yl)-1H-benzo[d]imidazole as an emitter displayed promising deep blue emission characteristics with a maximum luminance of 714 cd m−2, external quantum efficiency of 1.5%, CIE coordinates of (0.16, 0.05) at 100 cd m−2 and 100% color saturation.

 Please wait while we load your content...
Please wait while we load your content...